Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study
- PMID: 34600747
- PMCID: PMC8461292
- DOI: 10.1016/j.vaccine.2021.09.055
Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study
Abstract
Background: We assessed the humoral immune response of both ChAdOx1-nCOV (CovishieldTM) and BBV-152 (CovaxinTM) vaccines in Indian health care workers (HCW).
Methods: A Pan-India, Cross-sectional, Coronavirus Vaccine-induced Antibody Titre (COVAT) study was conducted that measured SARS-CoV-2 anti-spike binding antibody quantitatively, 21 days or more after the first and second dose of two vaccines in both severe acute respiratory syndrome (SARS-CoV-2) naïve and recovered HCW. Primary aim was to analyze antibody response (seropositivity rate, Geometric Mean Titre [GMT] and 95% Confidence Interval [CI]) following each dose of both vaccines and its correlation to age, sex, blood group, body mass index (BMI) and comorbidities. Here we report the results of anti-spike antibody response after first and two completed doses.
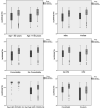
Results: Among the 515 HCW (305 Male, 210 Female) who took two doses of both vaccines, 95.0% showed seropositivity to anti-spike antibody. However, both seropositivity rate and GMT (95% CI) of anti-spike antibody was significantly higher in Covishield vs. Covaxin recipients (98.1 vs. 80.0%; 129.3 vs. 48.3 AU/mL; both p < 0.001). This difference persisted in 457 SARS-CoV-2 naïve and propensity-matched (age, sex and BMI) analysis of 116 participants. Age > 60-years, males, people with any comorbidities, and history of hypertension (HTN) had a significantly less anti-spike antibody GMT compared to age ≤ 60 years, females, no comorbidities and no HTN respectively, after the completion of two doses of either vaccine. Gender, presence of comorbidities, and vaccine type were independent predictors of antibody seropositivity rate and anti-spike antibody titre levels in multiple logistic and log transformed linear regression analysis. Both vaccine recipients had similar solicited mild to moderate adverse events and none had severe or unsolicited side effects.
Conclusions: Both vaccines elicited good immune response after two doses, although seropositivity rates and GMT of anti-spike antibody titre was significantly higher in Covishield compared to Covaxin recipients.
Keywords: Anti-spike antibody; Comorbidities; Immunogenicity; SARS-CoV-2; Vaccines.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. - PMC - PubMed
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. - PMC - PubMed
-
- Bharat Biotech Announces Phase 3 Results of COVAXIN®: India’s First COVID-19 Vaccine Demonstrates Interim Clinical Efficacy of 81%. covaxin-phase3-efficacy-results.pdf (bharatbiotech.com). Accessed on May 31, 2021.
-
- Singh AK, Phatak SR, Singh NK, et al. Antibody Response after First-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) amongst Health Care Workers in India: Preliminary Results of Cross-sectional Coronavirus Vaccine-induced Antibody Titre (COVAT) study. medRxiv preprint doi: 10.1101/2021.04.07.21255078. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

