Hemochromatosis classification: update and recommendations by the BIOIRON Society
- PMID: 34601591
- PMCID: PMC11022970
- DOI: 10.1182/blood.2021011338
Hemochromatosis classification: update and recommendations by the BIOIRON Society
Abstract
Hemochromatosis (HC) is a genetically heterogeneous disorder in which uncontrolled intestinal iron absorption may lead to progressive iron overload (IO) responsible for disabling and life-threatening complications such as arthritis, diabetes, heart failure, hepatic cirrhosis, and hepatocellular carcinoma. The recent advances in the knowledge of pathophysiology and molecular basis of iron metabolism have highlighted that HC is caused by mutations in at least 5 genes, resulting in insufficient hepcidin production or, rarely, resistance to hepcidin action. This has led to an HC classification based on different molecular subtypes, mainly reflecting successive gene discovery. This scheme was difficult to adopt in clinical practice and therefore needs revision. Here we present recommendations for unambiguous HC classification developed by a working group of the International Society for the Study of Iron in Biology and Medicine (BIOIRON Society), including both clinicians and basic scientists during a meeting in Heidelberg, Germany. We propose to deemphasize the use of the molecular subtype criteria in favor of a classification addressing both clinical issues and molecular complexity. Ferroportin disease (former type 4a) has been excluded because of its distinct phenotype. The novel classification aims to be of practical help whenever a detailed molecular characterization of HC is not readily available.
© 2022 by The American Society of Hematology.
Figures
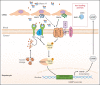


Comment in
-
Hemochromatosis redefined.Blood. 2022 May 19;139(20):3001-3002. doi: 10.1182/blood.2021014036. Blood. 2022. PMID: 35587868 No abstract available.
References
-
- Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139(2):393–408. 408 e391-392. - PubMed
-
- Barton JC, Bertoli LF, Rothenberg BE. Peripheral blood erythrocyte parameters in hemochromatosis: evidence for increased erythrocyte hemoglobin content. J Lab Clin Med. 2000;135(1):96–104. - PubMed
-
- Beutler E, Felitti V, Gelbart T, Waalen J. Haematological effects of the C282Y HFE mutation in homozygous and heterozygous states among subjects of northern and southern European ancestry. Br J Haematol. 2003;120(5):887–893. - PubMed
-
- Datz C, Haas T, Rinner H, Sandhofer F, Patsch W, Paulweber B. Heterozygosity for the C282Y mutation in the hemochromatosis gene is associated with increased serum iron, transferrin saturation, and hemoglobin in young women: a protective role against iron deficiency? Clin Chem. 1998;44(12):2429–2432. - PubMed
-
- McLaren CE, Barton JC, Gordeuk VR, et al. Hemochromatosis and Iron Overload Screening Study Research Investigators Determinants and characteristics of mean corpuscular volume and hemoglobin concentration in white HFE C282Y homozygotes in the hemochromatosis and iron overload screening study. Am J Hematol. 2007;82(10):898–905. - PubMed

