Expert opinion on monitoring symptomatic hereditary transthyretin-mediated amyloidosis and assessment of disease progression
- PMID: 34602081
- PMCID: PMC8489116
- DOI: 10.1186/s13023-021-01960-9
Expert opinion on monitoring symptomatic hereditary transthyretin-mediated amyloidosis and assessment of disease progression
Abstract
Background: Hereditary transthyretin-mediated amyloidosis, also known as ATTRv amyloidosis (v for variant), is a rare, autosomal dominant, fatal disease, in which systemic amyloid progressively impairs multiple organs, leading to disability and death. The recent approval of disease-modifying therapies offers the hope of stabilization or eventual reversal of disease progression, and yet highlights a lack of disease-management guidance. A multidisciplinary panel of expert clinicians from France and the US came to consensus on monitoring the disease and identifying progression through a clinical opinion questionnaire, a roundtable meeting, and multiple rounds of feedback.
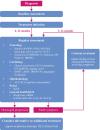
Monitoring disease and progression: A multidisciplinary team should monitor ATTRv amyloidosis disease course by assessing potential target organs at baseline and during follow-up for signs and symptoms of somatic and autonomic neuropathy, cardiac dysfunction and restrictive cardiomyopathy, and other manifestations. Variability in penetrance, symptoms, and course of ATTRv amyloidosis requires that all patients, regardless of variant status, undergo regular and standardized assessment in all these categories. Progression in ATTRv amyloidosis may be indicated by: worsening of several existing quantifiable symptoms or signs; the appearance of a new symptom; or the worsening of a single symptom that results in a meaningful functional impairment.
Conclusions: We suggest that a multisystem approach to monitoring the signs and symptoms of ATTRv amyloidosis best captures the course of the disease. We hope this work will help form the basis of further, consensus-based guidance for the treatment of ATTRv amyloidosis.
Keywords: ATTRv amyloidosis; Amyloid cardiomyopathies; Amyloid neuropathies; Diagnostic techniques and procedures; Disease progression; Familial; Transthyretin; hATTR amyloidosis.
© 2021. The Author(s).
Conflict of interest statement
DA acknowledges consultancy fees and institutional grants from Alnylam Pharmaceuticals and Pfizer Inc., and symposium honoraria from Pfizer Inc. outside the submitted work. VA reports consultancy fees from Pfizer Inc. and consultancy fees and institutional grants from Alnylam Pharmaceuticals outside the submitted work. JN-N’s institution received funding for clinical trials for Pfizer Inc., Akcea Therapeutics, and Eidos, and educational grants from Pfizer Inc., and received consulting income from Pfizer Inc., Eidos, Akcea Therapeutics, and Alnylam Pharmaceuticals outside the submitted work. MP reports consultancy and principal investigator fees from Alnylam Pharmaceuticals in relation to this work and from Alnylam Pharmaceuticals, Ionis Pharmaceuticals, and Pfizer Inc. outside the submitted work. NS reports funding for clinical trials for Pfizer, Akcea/Ionis, Alnylam, and Eidos, and consulting income from Pfizer, Akcea, and Alnylam. MSS acknowledges personal consultancy fees and symposium honoraria from Alnylam Pharmaceuticals and Pfizer Inc. outside the submitted work. The authors were not paid for their work developing the manuscript. The sponsor initially contacted the authors to ascertain their interest in writing the manuscript but had no role in the design, execution, interpretation, or writing of the study.
Figures
References
-
- Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep. 2014;11(1):50–57. - PubMed
-
- Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15(7):387–404. - PubMed
-
- Soprano DR, Herbert J, Soprano KJ, Schon EA, Goodman DS. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985;260(21):11793–11798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

