My Research Results: a program to facilitate return of clinically actionable genomic research findings
- PMID: 34602610
- PMCID: PMC8904822
- DOI: 10.1038/s41431-021-00973-z
My Research Results: a program to facilitate return of clinically actionable genomic research findings
Abstract
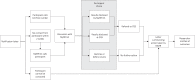
Researchers and research participants increasingly support returning clinically actionable genetic research findings to participants, but researchers may lack the skills and resources to do so. In response, a genetic counsellor-led program to facilitate the return of clinically actionable findings to research participants was developed to fill the identified gap in research practice and meet Australian research guidelines. A steering committee of experts reviewed relevant published literature and liaised with researchers, research participants and clinicians to determine the scope of the program, as well as the structure, protocols and infrastructure. A program called My Research Results (MyRR) was developed, staffed by genetic counsellors with input from the steering committee, infrastructure services and a genomic advisory committee. MyRR is available to Human Research Ethics Committee approved studies Australia-wide and comprises genetic counselling services to notify research participants of clinically actionable research findings, support for researchers with developing an ethical strategy for managing research findings and an online information platform. The results notification strategy is an evidence-based two-step model, which has been successfully used in other Australian studies. MyRR is a translational program supporting researchers and research participants to access clinically actionable research findings.
© 2021. The Author(s), under exclusive licence to European Society of Human Genetics.
Conflict of interest statement
The authors declare no competing interests.
Figures
Comment in
-
Invited Commentary on "My Research Results: a program to facilitate return of clinically actionable genomic research findings" by Willis et al.Eur J Hum Genet. 2022 Mar;30(3):256-257. doi: 10.1038/s41431-021-01003-8. Epub 2021 Nov 22. Eur J Hum Genet. 2022. PMID: 34803162 Free PMC article. No abstract available.
References
-
- Hart MR, Biesecker BB, Blout CL, Christensen KD, Amendola LM, Bergstrom KL, et al. Secondary findings from clinical genomic sequencing: prevalence, patient perspectives, family history assessment, and health-care costs from a multisite study. Genet Med. 2019;21:1100–10. doi: 10.1038/s41436-018-0308-x. - DOI - PMC - PubMed
-
- The National Health and Medical Research Council, the Australian Research Council, Universities Australia. National Statement on Ethical Conduct in Human Research. Commonwealth of Australia, Canberra: National Health and Medical Research Council; 2007 (Updated 2018).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous