Innovation in the discovery of the HIV-1 attachment inhibitor temsavir and its phosphonooxymethyl prodrug fostemsavir
- PMID: 34602806
- PMCID: PMC8476988
- DOI: 10.1007/s00044-021-02787-6
Innovation in the discovery of the HIV-1 attachment inhibitor temsavir and its phosphonooxymethyl prodrug fostemsavir
Abstract
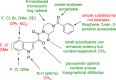
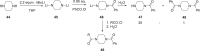
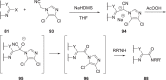
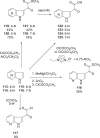
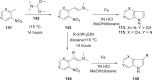
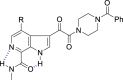
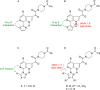
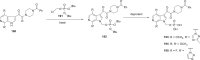
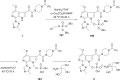
The discovery and development of fostemsavir (2), the tromethamine salt of the phosphonooxymethyl prodrug of temsavir (1), encountered significant challenges at many points in the preclinical and clinical development program that, in many cases, stimulated the implementation of innovative solutions in order to enable further progression. In the preclinical program, a range of novel chemistry methodologies were developed during the course of the discovery effort that enabled a thorough examination and definition of the HIV-1 attachment inhibitor (AI) pharmacophore. These discoveries helped to address the challenges associated with realizing a molecule with all of the properties necessary to successfully advance through development and this aspect of the program is the major focus of this retrospective. Although challenges and innovation are not unusual in drug discovery and development programs, the HIV-1 AI program is noteworthy not only because of the serial nature of the challenges encountered along the development path, but also because it resulted in a compound that remains the first and only example of a mechanistically novel class of HIV-1 inhibitor that is proving to be very beneficial for controlling virus levels in highly treatment-experienced HIV-1 infected patients.
Keywords: Fostemsavir; HIV-1 attachment inhibitors; Indole-3-gyloxamide; Prodrug; Synthetic methodology; Temsavir.
© The Author(s) 2021.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures
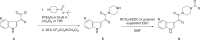
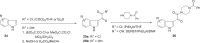
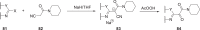
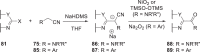
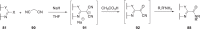
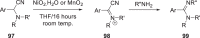


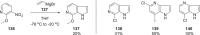

Similar articles
-
Viral Drug Resistance Through 48 Weeks, in a Phase 2b, Randomized, Controlled Trial of the HIV-1 Attachment Inhibitor Prodrug, Fostemsavir.J Acquir Immune Defic Syndr. 2018 Mar 1;77(3):299-307. doi: 10.1097/QAI.0000000000001602. J Acquir Immune Defic Syndr. 2018. PMID: 29206721 Free PMC article. Clinical Trial.
-
Inhibitors of HIV-1 Attachment: The Discovery and Development of Temsavir and its Prodrug Fostemsavir.J Med Chem. 2018 Jan 11;61(1):62-80. doi: 10.1021/acs.jmedchem.7b01337. Epub 2017 Dec 22. J Med Chem. 2018. PMID: 29271653
-
Discovery of the Human Immunodeficiency Virus Type 1 (HIV-1) Attachment Inhibitor Temsavir and Its Phosphonooxymethyl Prodrug Fostemsavir.J Med Chem. 2018 Jul 26;61(14):6308-6327. doi: 10.1021/acs.jmedchem.8b00759. Epub 2018 Jul 13. J Med Chem. 2018. PMID: 29920093
-
Fostemsavir resistance in clinical context: a narrative review.Ther Adv Infect Dis. 2025 Mar 24;12:20499361251325103. doi: 10.1177/20499361251325103. eCollection 2025 Jan-Dec. Ther Adv Infect Dis. 2025. PMID: 40145022 Free PMC article. Review.
-
Small Molecule HIV-1 Attachment Inhibitors: Discovery, Mode of Action and Structural Basis of Inhibition.Viruses. 2021 May 6;13(5):843. doi: 10.3390/v13050843. Viruses. 2021. PMID: 34066522 Free PMC article. Review.
Cited by
-
Methoxy group: a non-lipophilic "scout" for protein pocket finding.Future Med Chem. 2025 May;17(9):983-985. doi: 10.1080/17568919.2025.2485865. Epub 2025 Apr 3. Future Med Chem. 2025. PMID: 40176768 No abstract available.
-
Drug Discovery and Exploration of Heterocycles for the Development of Anti-HIV Agents.Infect Disord Drug Targets. 2025;25(3):e18715265290911. doi: 10.2174/0118715265290911240611072422. Infect Disord Drug Targets. 2025. PMID: 39185647 Review.
-
Fresh Baked: An Overview of Newly FDA-Approved Drugs for Dermatological Usage.Indian J Dermatol. 2023 Nov-Dec;68(6):707-720. doi: 10.4103/ijd.IJD_809_23. Epub 2024 Jan 9. Indian J Dermatol. 2023. PMID: 38371570 Free PMC article.
-
Current drugs for HIV-1: from challenges to potential in HIV/AIDS.Front Pharmacol. 2023 Oct 26;14:1294966. doi: 10.3389/fphar.2023.1294966. eCollection 2023. Front Pharmacol. 2023. PMID: 37954841 Free PMC article. Review.
-
Antiviral Agents: Structural Basis of Action and Rational Design.Subcell Biochem. 2024;105:745-784. doi: 10.1007/978-3-031-65187-8_20. Subcell Biochem. 2024. PMID: 39738962 Review.
References
-
- Seval N, Frank C, Koza M. Fostemsavir for the treatment of HIV. Expert Rev Anti Infect Ther. 2021; 10.1080/14787210.2021.1865801. - PubMed
-
- Blair W, Spicer TP. HIV-1 reporter viruses and their use in assaying anti-viral compounds. World Patent Application, WO-2001/096610, December 20th, 2001.
Publication types
LinkOut - more resources
Full Text Sources
