Neu Perspectives, Therapies, and Challenges for Metastatic HER2-Positive Breast Cancer
- PMID: 34602823
- PMCID: PMC8481821
- DOI: 10.2147/BCTT.S288344
Neu Perspectives, Therapies, and Challenges for Metastatic HER2-Positive Breast Cancer
Abstract
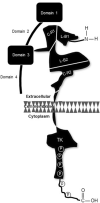
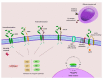
Even though gene amplification or protein overexpression occurs in approximately one-fifth of all breast cancers, the discovery of HER2 has, nevertheless, had profound implications for the disease. Indeed, the characterization of the receptor resulted in a number of significant advances. Structurally, unique features provided avenues for the development of numerous compounds with target-specificity; molecularly, biological constructs revealed a highly complex, internal signal transduction pathway with regulatory effects on tumor proliferation, survival, and perhaps, even resistance; and clinically, disease outcomes manifested its predictive and prognostic value. Yet despite the receptor's utility, the beneficial effects are diminished by tumor recurrence after neo- or adjuvant therapy as well as losses resulting from the inability to cure patients with metastatic disease. What these observations suggest is that while tumor response may be partially linked to uncoupling cell surface message reception and nuclear gene expression, as well as recruitment of the innate immune system, disease progression and/or resistance may involve a reprogrammable signaling mainframe that elicits alternative growth and survival signals. This review attempts to meld current perceptions related to HER2-positive metastatic breast cancer with particular attention to current biological insights and therapeutic challenges.
Keywords: ErbB; Fam-trastuzumab deruxtecan; HER2; PHESGO; ado-trastuzumab emtansine; lapatinib; margetuximab; neratinib; neu; pertuzumab; trastuzumab; tucatinib.
© 2021 Salkeni et al.
Conflict of interest statement
The authors do not have any relationship, financial or otherwise (ie, support in the form of employment, consultancies, honoraria, stock ownership and options, expert testimony, grants or patents received or pending, or royalties) with manufacturers of all agents described that influenced the writing of this manuscript.
Figures
Similar articles
-
Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation.Oncologist. 2021 Aug;26(8):635-639. doi: 10.1002/onco.13715. Epub 2021 Mar 11. Oncologist. 2021. PMID: 33559918 Free PMC article.
-
Novel HER2-targeted therapies for HER2-positive metastatic breast cancer.Cancer. 2020 Oct 1;126(19):4278-4288. doi: 10.1002/cncr.33102. Epub 2020 Jul 28. Cancer. 2020. PMID: 32721042 Review.
-
Systemic therapy for metastatic HER2-positive breast cancer.Semin Oncol. 2020 Oct;47(5):259-269. doi: 10.1053/j.seminoncol.2020.07.008. Epub 2020 Aug 18. Semin Oncol. 2020. PMID: 32896428 Review.
-
Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate.Ann Pharmacother. 2014 Nov;48(11):1484-93. doi: 10.1177/1060028014545354. Epub 2014 Jul 31. Ann Pharmacother. 2014. PMID: 25082874 Review.
-
Targeting HER2 in Breast Cancer: Latest Developments on Treatment Sequencing and the Introduction of Biosimilars.Drugs. 2020 Nov;80(17):1811-1830. doi: 10.1007/s40265-020-01411-y. Drugs. 2020. PMID: 33021725 Review.
Cited by
-
Comparative Assessment of Quantification Methods for Tumor Tissue Phosphoproteomics.Anal Chem. 2022 Aug 9;94(31):10893-10906. doi: 10.1021/acs.analchem.2c01036. Epub 2022 Jul 26. Anal Chem. 2022. PMID: 35880733 Free PMC article.
-
Nanoparticles (NPs)-mediated systemic mRNA delivery to reverse trastuzumab resistance for effective breast cancer therapy.Acta Pharm Sin B. 2023 Mar;13(3):955-966. doi: 10.1016/j.apsb.2022.09.021. Epub 2022 Oct 4. Acta Pharm Sin B. 2023. PMID: 36970191 Free PMC article.
References
-
- Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981;290(5803):261–264. doi:10:1038/290261a0 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

