Glial Chloride Homeostasis Under Transient Ischemic Stress
- PMID: 34602981
- PMCID: PMC8481871
- DOI: 10.3389/fncel.2021.735300
Glial Chloride Homeostasis Under Transient Ischemic Stress
Abstract
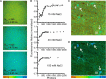
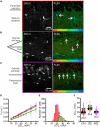
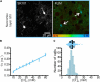
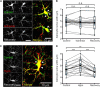
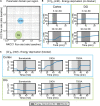
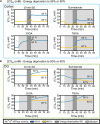
High water permeabilities permit rapid adjustments of glial volume upon changes in external and internal osmolarity, and pathologically altered intracellular chloride concentrations ([Cl-]int) and glial cell swelling are often assumed to represent early events in ischemia, infections, or traumatic brain injury. Experimental data for glial [Cl-]int are lacking for most brain regions, under normal as well as under pathological conditions. We measured [Cl-]int in hippocampal and neocortical astrocytes and in hippocampal radial glia-like (RGL) cells in acute murine brain slices using fluorescence lifetime imaging microscopy with the chloride-sensitive dye MQAE at room temperature. We observed substantial heterogeneity in baseline [Cl-]int, ranging from 14.0 ± 2.0 mM in neocortical astrocytes to 28.4 ± 3.0 mM in dentate gyrus astrocytes. Chloride accumulation by the Na+-K+-2Cl- cotransporter (NKCC1) and chloride outward transport (efflux) through K+-Cl- cotransporters (KCC1 and KCC3) or excitatory amino acid transporter (EAAT) anion channels control [Cl-]int to variable extent in distinct brain regions. In hippocampal astrocytes, blocking NKCC1 decreased [Cl-]int, whereas KCC or EAAT anion channel inhibition had little effect. In contrast, neocortical astrocytic or RGL [Cl-]int was very sensitive to block of chloride outward transport, but not to NKCC1 inhibition. Mathematical modeling demonstrated that higher numbers of NKCC1 and KCC transporters can account for lower [Cl-]int in neocortical than in hippocampal astrocytes. Energy depletion mimicking ischemia for up to 10 min did not result in pronounced changes in [Cl-]int in any of the tested glial cell types. However, [Cl-]int changes occurred under ischemic conditions after blocking selected anion transporters. We conclude that stimulated chloride accumulation and chloride efflux compensate for each other and prevent glial swelling under transient energy deprivation.
Keywords: K-Cl cotransporters; Na-K-2Cl cotransporter; chemical stress mimicking ischemia; excitatory amino acid transporters; fluorescence lifetime imaging microscopy; intracellular chloride concentrations.
Copyright © 2021 Engels, Kalia, Rahmati, Petersilie, Kovermann, van Putten, Rose, Meijer, Gensch and Fahlke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Glutamate transporter-associated anion channels adjust intracellular chloride concentrations during glial maturation.Glia. 2017 Feb;65(2):388-400. doi: 10.1002/glia.23098. Epub 2016 Nov 18. Glia. 2017. PMID: 27859594
-
Optical imaging reveals cation--Cl(-) cotransporter-mediated transient rapid decrease in intracellular Cl(-) concentration induced by oxygen--glucose deprivation in rat neocortical slices.Neurosci Res. 2001 Mar;39(3):269-80. doi: 10.1016/s0168-0102(00)00221-2. Neurosci Res. 2001. PMID: 11248366
-
Roles of the cation-chloride cotransporters in neurological disease.Nat Clin Pract Neurol. 2008 Sep;4(9):490-503. doi: 10.1038/ncpneuro0883. Nat Clin Pract Neurol. 2008. PMID: 18769373 Review.
-
Disruption of an EAAT-Mediated Chloride Channel in a Drosophila Model of Ataxia.J Neurosci. 2016 Jul 20;36(29):7640-7. doi: 10.1523/JNEUROSCI.0197-16.2016. J Neurosci. 2016. PMID: 27445142 Free PMC article.
-
The signaling role for chloride in the bidirectional communication between neurons and astrocytes.Neurosci Lett. 2019 Jan 10;689:33-44. doi: 10.1016/j.neulet.2018.01.012. Epub 2018 Jan 9. Neurosci Lett. 2019. PMID: 29329909 Free PMC article. Review.
Cited by
-
The severity of SLC1A2-associated neurodevelopmental disorders correlates with transporter dysfunction.EBioMedicine. 2025 Apr;114:105648. doi: 10.1016/j.ebiom.2025.105648. Epub 2025 Apr 1. EBioMedicine. 2025. PMID: 40174554 Free PMC article.
-
Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons.Cells. 2022 Aug 9;11(16):2470. doi: 10.3390/cells11162470. Cells. 2022. PMID: 36010547 Free PMC article.
-
Changes in Astroglial K+ upon Brief Periods of Energy Deprivation in the Mouse Neocortex.Int J Mol Sci. 2022 Apr 27;23(9):4836. doi: 10.3390/ijms23094836. Int J Mol Sci. 2022. PMID: 35563238 Free PMC article.
-
In Vitro Effect of 9,9'-Norharmane Dimer against Herpes Simplex Viruses.Int J Mol Sci. 2024 May 2;25(9):4966. doi: 10.3390/ijms25094966. Int J Mol Sci. 2024. PMID: 38732185 Free PMC article.
-
Epileptic activity triggers rapid ROCK1-dependent astrocyte morphology changes.Glia. 2024 Mar;72(3):643-659. doi: 10.1002/glia.24495. Epub 2023 Nov 30. Glia. 2024. PMID: 38031824 Free PMC article.
References
-
- Abrahamsen B., Schneider N., Erichsen M. N., Huynh T. H., Fahlke C., Bunch L., et al. (2013). Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J. Neurosci. 33 1068–1087. 10.1523/jneurosci.3396-12.2013 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

