Functional Metabolic Mapping Reveals Highly Active Branched-Chain Amino Acid Metabolism in Human Astrocytes, Which Is Impaired in iPSC-Derived Astrocytes in Alzheimer's Disease
- PMID: 34603012
- PMCID: PMC8484639
- DOI: 10.3389/fnagi.2021.736580
Functional Metabolic Mapping Reveals Highly Active Branched-Chain Amino Acid Metabolism in Human Astrocytes, Which Is Impaired in iPSC-Derived Astrocytes in Alzheimer's Disease
Abstract
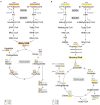
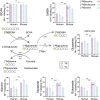
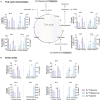
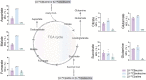
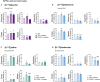
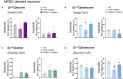
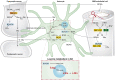
The branched-chain amino acids (BCAAs) leucine, isoleucine, and valine are important nitrogen donors for synthesis of glutamate, the main excitatory neurotransmitter in the brain. The glutamate carbon skeleton originates from the tricarboxylic acid (TCA) cycle intermediate α-ketoglutarate, while the amino group is derived from nitrogen donors such as the BCAAs. Disturbances in neurotransmitter homeostasis, mainly of glutamate, are strongly implicated in the pathophysiology of Alzheimer's disease (AD). The divergent BCAA metabolism in different cell types of the human brain is poorly understood, and so is the involvement of astrocytic and neuronal BCAA metabolism in AD. The goal of this study is to provide the first functional characterization of BCAA metabolism in human brain tissue and to investigate BCAA metabolism in AD pathophysiology using astrocytes and neurons derived from human-induced pluripotent stem cells (hiPSCs). Mapping of BCAA metabolism was performed using mass spectrometry and enriched [15N] and [13C] isotopes of leucine, isoleucine, and valine in acutely isolated slices of surgically resected cerebral cortical tissue from human brain and in hiPSC-derived brain cells carrying mutations in either amyloid precursor protein (APP) or presenilin-1 (PSEN-1). We revealed that both human astrocytes of acutely isolated cerebral cortical slices and hiPSC-derived astrocytes were capable of oxidatively metabolizing the carbon skeleton of BCAAs, particularly to support glutamine synthesis. Interestingly, hiPSC-derived astrocytes with APP and PSEN-1 mutations exhibited decreased amino acid synthesis of glutamate, glutamine, and aspartate derived from leucine metabolism. These results clearly demonstrate that there is an active BCAA metabolism in human astrocytes, and that leucine metabolism is selectively impaired in astrocytes derived from the hiPSC models of AD. This impairment in astrocytic BCAA metabolism may contribute to neurotransmitter and energetic imbalances in the AD brain.
Keywords: AD; BCAA; astrocytes; energy metabolism; glutamate; glutamine; induced pluripotent stem cell; neuron.
Copyright © 2021 Salcedo, Andersen, Vinten, Pinborg, Waagepetersen, Freude and Aldana.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Increased glucose metabolism and impaired glutamate transport in human astrocytes are potential early triggers of abnormal extracellular glutamate accumulation in hiPSC-derived models of Alzheimer's disease.J Neurochem. 2024 May;168(5):822-840. doi: 10.1111/jnc.16014. Epub 2023 Dec 8. J Neurochem. 2024. PMID: 38063257
-
Enhanced cerebral branched-chain amino acid metabolism in R6/2 mouse model of Huntington's disease.Cell Mol Life Sci. 2019 Jun;76(12):2449-2461. doi: 10.1007/s00018-019-03051-2. Epub 2019 Mar 4. Cell Mol Life Sci. 2019. PMID: 30830240 Free PMC article.
-
Downregulation of GABA Transporter 3 (GAT3) is Associated with Deficient Oxidative GABA Metabolism in Human Induced Pluripotent Stem Cell-Derived Astrocytes in Alzheimer's Disease.Neurochem Res. 2021 Oct;46(10):2676-2686. doi: 10.1007/s11064-021-03276-3. Epub 2021 Mar 12. Neurochem Res. 2021. PMID: 33710537
-
Brain metabolism of branched-chain amino acids.Glia. 1997 Sep;21(1):92-8. doi: 10.1002/(sici)1098-1136(199709)21:1<92::aid-glia10>3.0.co;2-w. Glia. 1997. PMID: 9298851 Review.
-
Insulin Resistance and Impaired Branched-Chain Amino Acid Metabolism in Alzheimer's Disease.J Alzheimers Dis. 2023;93(3):847-862. doi: 10.3233/JAD-221147. J Alzheimers Dis. 2023. PMID: 37125547 Review.
Cited by
-
Brain Energy Metabolism: Astrocytes in Neurodegenerative Diseases.CNS Neurosci Ther. 2023 Jan;29(1):24-36. doi: 10.1111/cns.13982. Epub 2022 Oct 3. CNS Neurosci Ther. 2023. PMID: 36193573 Free PMC article. Review.
-
Ketogenic diet therapy for pediatric epilepsy is associated with alterations in the human gut microbiome that confer seizure resistance in mice.Cell Rep. 2023 Dec 26;42(12):113521. doi: 10.1016/j.celrep.2023.113521. Epub 2023 Dec 8. Cell Rep. 2023. PMID: 38070135 Free PMC article.
-
Astrocytes in Neurodegeneration: Inspiration From Genetics.Front Neurosci. 2022 Jun 24;16:882316. doi: 10.3389/fnins.2022.882316. eCollection 2022. Front Neurosci. 2022. PMID: 35812232 Free PMC article. Review.
-
Pyrroloquinoline quinone drives ATP synthesis in vitro and in vivo and provides retinal ganglion cell neuroprotection.Acta Neuropathol Commun. 2023 Sep 8;11(1):146. doi: 10.1186/s40478-023-01642-6. Acta Neuropathol Commun. 2023. PMID: 37684640 Free PMC article.
-
Branched Chain Amino Acid Metabolism in Developmental Brain Injury: Putative Mechanisms and Therapeutic Potential.Dev Neurosci. 2025 Mar 11:1-15. doi: 10.1159/000545099. Online ahead of print. Dev Neurosci. 2025. PMID: 40068641 Review.
References
-
- Andersen J. V., Christensen S., Westi E., Diaz-delCastillo M., Tanila H., Schousboe A., et al. . (2021a). Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer's disease. Neurobiol. Dis. 148:105198. 10.1016/j.nbd.2020.105198 - DOI - PubMed
LinkOut - more resources
Full Text Sources

