The Efficacy and Safety of Infliximab in Refractory Noninfectious Uveitis: A Meta-Analysis of Observational Studies
- PMID: 34603013
- PMCID: PMC8481770
- DOI: 10.3389/fphar.2021.620340
The Efficacy and Safety of Infliximab in Refractory Noninfectious Uveitis: A Meta-Analysis of Observational Studies
Abstract
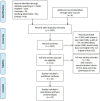
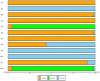
Background: Although infliximab has been recommended for the second-line treatment of seronegative spondyloarthropathy- or juvenile idiopathic arthritis-related uveitis, the issue of its systemic efficacy and safety in a broader diversity of refractory noninfectious uveitis is debatable. To assess the short-term and relatively long-term efficacy of infliximab in refractory noninfectious uveitis, we performed a systematic review and meta-analysis of observational studies. Methods: PubMed, Cochrane Library, EMBASE, and Wanfang Med Online were systematically searched from January 2005 to March 2020. Two investigators independently assessed eligibility. Data were independently collected by two investigators. The pooled proportions were estimated with patients for intraocular inflammation control and improvement of visual acuity. Pooled proportions with 95% credible intervals were computed. Study homogeneity was investigated using I 2 statistics to quantify the percentage of variation across studies. To pool the results, the Mantel-Haenszel fixed-effects or random-effects models were used. Results: Of 2316 studies identified, 16 unique studies with 509 unique participants were included in the meta-analysis. The pooled proportions of intraocular inflammation control reached 92% (95% CI: 87%-98%; I 2: 1%; p=0.42) and 95% (95% CI: 93%-97%; I 2: 0%; p=0.91) in groups of ≤6- and ≥12-month follow-up durations. During the relatively long follow-up period, the pooled proportions of maintaining visual acuity stable or increasing at least one line reached 99% (95% CI: 96%-100%; I 2: 0%; p=0.54) in the involved eyes. The corticosteroid-sparing effect of infliximab was also well demonstrated, with the proportion of corticosteroid-sparing success reaching 85.5% (112/131). Besides, about serious adverse events, 2.6% (13/500) of patients experienced hypersensitivity reactions, 2.4% (12/500) of patients experienced serious infections, 1.8% (9/500) of patients experienced autoimmune diseases, and 0.6% (3/500) of patients experienced neoplasia. Conclusions: This meta-analysis provided evidence that infliximab might be a promising choice in controlling inflammatory activity, gaining visual acuity, and sparing corticosteroid use with relatively few side effects when applied in treating refractory noninfectious uveitis. Systematic Review Registration: [website], identifier [registration number].
Keywords: anti-TNF-α; infliximab; noninfectious uveitis; refractory; uveitis treatment.
Copyright © 2021 Xiong, Liu, Chen, Yang, Xiong, Shuai, He, Guo, Zhang, Yang, Cui and Shuai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Efficacy and safety of adalimumab in the treatment of non-infectious uveitis: a meta-analysis and systematic review.Drug Des Devel Ther. 2018 Jul 4;12:2005-2016. doi: 10.2147/DDDT.S160431. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30013320 Free PMC article.
-
Outcomes of treatment with sirolimus for non-infectious uveitis: a meta-analysis and systematic review.Clin Ophthalmol. 2019 Apr 18;13:649-669. doi: 10.2147/OPTH.S198401. eCollection 2019. Clin Ophthalmol. 2019. PMID: 31114144 Free PMC article. Review.
-
Infliximab Versus Adalimumab in the Treatment of Refractory Inflammatory Uveitis: A Multicenter Study From the French Uveitis Network.Arthritis Rheumatol. 2016 Jun;68(6):1522-30. doi: 10.1002/art.39667. Arthritis Rheumatol. 2016. PMID: 27015607
-
Retrospective case review of pediatric patients with uveitis treated with infliximab.Ophthalmology. 2006 Feb;113(2):308-14. doi: 10.1016/j.ophtha.2005.09.037. Epub 2006 Jan 10. Ophthalmology. 2006. PMID: 16406545
-
Long-Term Outcomes of Treatment with Biological Agents in Eyes with Refractory, Active, Noninfectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis.Ophthalmology. 2020 Mar;127(3):410-416. doi: 10.1016/j.ophtha.2019.08.031. Epub 2019 Sep 6. Ophthalmology. 2020. PMID: 31607412
Cited by
-
Saudi Clinical Practice Guidelines for Management of Axial Spondyloarthritis Disease.Rheumatol Ther. 2025 Jul 8. doi: 10.1007/s40744-025-00779-1. Online ahead of print. Rheumatol Ther. 2025. PMID: 40629208
-
Uveitis in the Pediatric Population and Therapeutic Management: A Current Literature Review.Children (Basel). 2024 Jun 25;11(7):769. doi: 10.3390/children11070769. Children (Basel). 2024. PMID: 39062219 Free PMC article. Review.
-
Guidelines for the standardized diagnosis and treatment of non-specific orbital inflammation (2024).Int J Ophthalmol. 2024 Dec 18;17(12):2203-2213. doi: 10.18240/ijo.2024.12.07. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39697897 Free PMC article.
-
The efficacy and safety of TNF inhibitor (golimumab) as salvage treatment in patients with refractory noninfectious uveitis.Inflammopharmacology. 2022 Aug;30(4):1363-1368. doi: 10.1007/s10787-022-01019-6. Epub 2022 Jul 8. Inflammopharmacology. 2022. PMID: 35802282
References
-
- Angeles-Han S. T., Ringold S., Beukelman T., Lovell D., Cuello C. A., Becker M. L., et al. (2019). 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Rheumatol. 71, 864–877. 10.1002/acr.2387110.1002/art.40885 - DOI - PMC - PubMed
-
- Giardina A., Ferrante A., Ciccia F., Vadalà M., Giardina E., Triolo G. (2011). One Year Study of Efficacy and Safety of Infliximab in the Treatment of Patients with Ocular and Neurological Behçet's Disease Refractory to Standard Immunosuppressive Drugs. Rheumatol. Int. 31, 33–37. 10.1007/s00296-009-1213-z - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

