Erythropoietin Non-hematopoietic Tissue Response and Regulation of Metabolism During Diet Induced Obesity
- PMID: 34603036
- PMCID: PMC8479821
- DOI: 10.3389/fphar.2021.725734
Erythropoietin Non-hematopoietic Tissue Response and Regulation of Metabolism During Diet Induced Obesity
Abstract
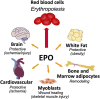
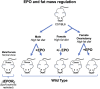
Erythropoietin (EPO) receptor (EPOR) determines EPO response. High level EPOR on erythroid progenitor cells gives rise to EPO regulated production of red blood cells. Animal models provide evidence for EPO activity in non-hematopoietic tissue mediated by EPOR expression. Beyond erythropoiesis, EPO activity includes neuroprotection in brain ischemia and trauma, endothelial nitric oxide production and cardioprotection, skeletal muscle wound healing, and context dependent bone remodeling affecting bone repair or bone loss. This review highlights examples of EPO protective activity in select non-hematopoietic tissue with emphasis on metabolic response mediated by EPOR expression in fat and brain and sex-specific regulation of fat mass and inflammation associated with diet induced obesity. Endogenous EPO maintains glucose and insulin tolerance and protects against fat mass accumulation and inflammation. Accompanying the increase in erythropoiesis with EPO treatment is improved glucose tolerance and insulin response. During high fat diet feeding, EPO also decreases fat mass accumulation in male mice. The increased white adipose tissue inflammation and macrophage infiltration associated with diet induced obesity are also reduced with EPO treatment with a shift toward an anti-inflammatory state and decreased inflammatory cytokine production. In female mice the protective effect of estrogen against obesity supersedes EPO regulation of fat mass and inflammation, and requires estrogen receptor alpha activity. In brain, EPOR expression in the hypothalamus localizes to proopiomelanocortin neurons in the arcuate nucleus that promotes a lean phenotype. EPO stimulation of proopiomelanocortin neurons increases STAT3 signaling and production of proopiomelanocortin. Cerebral EPO contributes to metabolic response, and elevated brain EPO reduces fat mass and hypothalamus inflammation during diet induced obesity in male mice without affecting EPO stimulated erythropoiesis. Ovariectomy abrogates the sex-specific metabolic response of brain EPO. The sex-dimorphic EPO metabolic response associated with fat mass accumulation and inflammation during diet induced obesity provide evidence for crosstalk between estrogen and EPO in their anti-obesity potential in female mice mediated in part via tissue specific response in brain and white adipose tissue. Endogenous and exogenous EPO response in non-hematopoietic tissue demonstrated in animal models suggests additional activity by which EPO treatment may affect human health beyond increased erythropoiesis.
Keywords: erythropoietin; erythropoietin receptor; gender-specific; hypothalamus; inflammation; microglial; obesity.
Copyright © 2021 Dey, Lee and Noguchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

