Therapeutic Drugs and Devices for Tackling Ocular Hypertension and Glaucoma, and Need for Neuroprotection and Cytoprotective Therapies
- PMID: 34603044
- PMCID: PMC8484316
- DOI: 10.3389/fphar.2021.729249
Therapeutic Drugs and Devices for Tackling Ocular Hypertension and Glaucoma, and Need for Neuroprotection and Cytoprotective Therapies
Abstract
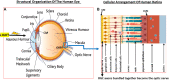
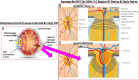
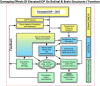
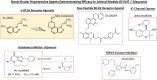
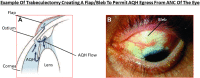
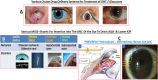
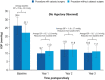
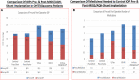
Damage to the optic nerve and the death of associated retinal ganglion cells (RGCs) by elevated intraocular pressure (IOP), also known as glaucoma, is responsible for visual impairment and blindness in millions of people worldwide. The ocular hypertension (OHT) and the deleterious mechanical forces it exerts at the back of the eye, at the level of the optic nerve head/optic disc and lamina cribosa, is the only modifiable risk factor associated with glaucoma that can be treated. The elevated IOP occurs due to the inability of accumulated aqueous humor (AQH) to egress from the anterior chamber of the eye due to occlusion of the major outflow pathway, the trabecular meshwork (TM) and Schlemm's canal (SC). Several different classes of pharmaceutical agents, surgical techniques and implantable devices have been developed to lower and control IOP. First-line drugs to promote AQH outflow via the uveoscleral outflow pathway include FP-receptor prostaglandin (PG) agonists (e.g., latanoprost, travoprost and tafluprost) and a novel non-PG EP2-receptor agonist (omidenepag isopropyl, Eybelis®). TM/SC outflow enhancing drugs are also effective ocular hypotensive agents (e.g., rho kinase inhibitors like ripasudil and netarsudil; and latanoprostene bunod, a conjugate of a nitric oxide donor and latanoprost). One of the most effective anterior chamber AQH microshunt devices is the Preserflo® microshunt which can lower IOP down to 10-13 mmHg. Other IOP-lowering drugs and devices on the horizon will be also discussed. Additionally, since elevated IOP is only one of many risk factors for development of glaucomatous optic neuropathy, a treatise of the role of inflammatory neurodegeneration of the optic nerve and retinal ganglion cells and appropriate neuroprotective strategies to mitigate this disease will also be reviewed and discussed.
Keywords: aqueous humor; drug discovery; glaucoma; intraocular pressur; neurodegenaration; neuroprotection; pharmacology.
Copyright © 2021 Sharif.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Recently Approved Drugs for Lowering and Controlling Intraocular Pressure to Reduce Vision Loss in Ocular Hypertensive and Glaucoma Patients.Pharmaceuticals (Basel). 2023 May 26;16(6):791. doi: 10.3390/ph16060791. Pharmaceuticals (Basel). 2023. PMID: 37375739 Free PMC article. Review.
-
FP and EP2 prostanoid receptor agonist drugs and aqueous humor outflow devices for treating ocular hypertension and glaucoma.Exp Eye Res. 2023 Apr;229:109415. doi: 10.1016/j.exer.2023.109415. Epub 2023 Feb 18. Exp Eye Res. 2023. PMID: 36803996 Review.
-
Human experience and efficacy of omidenepag isopropyl (Eybelis®; Omlonti®): Discovery to approval of the novel non-prostaglandin EP2-receptor-selective agonist ocular hypotensive drug.Curr Opin Pharmacol. 2024 Feb;74:102426. doi: 10.1016/j.coph.2023.102426. Epub 2024 Jan 1. Curr Opin Pharmacol. 2024. PMID: 38168596 Review.
-
The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies.J Ocul Pharmacol Ther. 2018 Jan/Feb;34(1-2):52-60. doi: 10.1089/jop.2016.0188. Epub 2017 Aug 7. J Ocul Pharmacol Ther. 2018. PMID: 28783422 Free PMC article. Review.
-
Gene therapies and gene product-based drug candidates for normalizing and preserving tissue functions in animal models of ocular hypertension and glaucoma.Mol Aspects Med. 2023 Dec;94:101218. doi: 10.1016/j.mam.2023.101218. Epub 2023 Nov 15. Mol Aspects Med. 2023. PMID: 37976898 Review.
Cited by
-
Development of Carvedilol Nanoformulation-Loaded Poloxamer-Based In Situ Gel for the Management of Glaucoma.Gels. 2023 Dec 4;9(12):952. doi: 10.3390/gels9120952. Gels. 2023. PMID: 38131938 Free PMC article.
-
scAAV2-Mediated Expression of Thioredoxin 2 and C3 Transferase Prevents Retinal Ganglion Cell Death and Lowers Intraocular Pressure in a Mouse Model of Glaucoma.Int J Mol Sci. 2023 Nov 13;24(22):16253. doi: 10.3390/ijms242216253. Int J Mol Sci. 2023. PMID: 38003443 Free PMC article.
-
Recently Approved Drugs for Lowering and Controlling Intraocular Pressure to Reduce Vision Loss in Ocular Hypertensive and Glaucoma Patients.Pharmaceuticals (Basel). 2023 May 26;16(6):791. doi: 10.3390/ph16060791. Pharmaceuticals (Basel). 2023. PMID: 37375739 Free PMC article. Review.
-
In vitro protective effect of recombinant prominin-1 combined with microRNA-29b on N-methyl-D-aspartate-induced excitotoxicity in retinal ganglion cells.Int J Ophthalmol. 2023 Nov 18;16(11):1746-1755. doi: 10.18240/ijo.2023.11.03. eCollection 2023. Int J Ophthalmol. 2023. PMID: 38028520 Free PMC article.
-
Targeted Metabolomics Shows That the Level of Glutamine, Kynurenine, Acyl-Carnitines and Lysophosphatidylcholines Is Significantly Increased in the Aqueous Humor of Glaucoma Patients.Front Med (Lausanne). 2022 Jul 22;9:935084. doi: 10.3389/fmed.2022.935084. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35935793 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

