Role of Intra- and Extracellular Lipid Signals in Cancer Stemness and Potential Therapeutic Strategy
- PMID: 34603046
- PMCID: PMC8479196
- DOI: 10.3389/fphar.2021.730751
Role of Intra- and Extracellular Lipid Signals in Cancer Stemness and Potential Therapeutic Strategy
Abstract
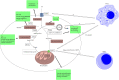
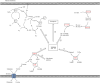
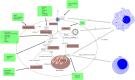
Accumulating evidence showed that cancer stem cells (CSCs) play significant roles in cancer initiation, resistance to therapy, recurrence and metastasis. Cancer stem cells possess the ability of self-renewal and can initiate tumor growth and avoid lethal factors through flexible metabolic reprogramming. Abnormal lipid metabolism has been reported to be involved in the cancer stemness and promote the development of cancer. Lipid metabolism includes lipid uptake, lipolysis, fatty acid oxidation, de novo lipogenesis, and lipid desaturation. Abnormal lipid metabolism leads to ferroptosis of CSCs. In this review, we comprehensively summarized the role of intra- and extracellular lipid signals in cancer stemness, and explored the feasibility of using lipid metabolism-related treatment strategies for future cancer.
Keywords: cancer stem cell; ferroptosis; lipid metabolism; therapeutic target; tumor environment.
Copyright © 2021 Hu, Zhang, Chen, Shen, Jiang, Sun and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Emerging role of lipid metabolism alterations in Cancer stem cells.J Exp Clin Cancer Res. 2018 Jun 15;37(1):118. doi: 10.1186/s13046-018-0784-5. J Exp Clin Cancer Res. 2018. PMID: 29907133 Free PMC article. Review.
-
Abnormal lipid synthesis as a therapeutic target for cancer stem cells.World J Stem Cells. 2022 Feb 26;14(2):146-162. doi: 10.4252/wjsc.v14.i2.146. World J Stem Cells. 2022. PMID: 35432735 Free PMC article. Review.
-
Aberrant lipid metabolism as an emerging therapeutic strategy to target cancer stem cells.Stem Cells. 2020 Jan;38(1):6-14. doi: 10.1002/stem.3101. Epub 2019 Oct 31. Stem Cells. 2020. PMID: 31648395 Review.
-
Lipid metabolism of cancer stem cells.Oncol Lett. 2022 Apr;23(4):119. doi: 10.3892/ol.2022.13239. Epub 2022 Feb 9. Oncol Lett. 2022. PMID: 35261633 Free PMC article. Review.
-
Targeting autophagy and lipid metabolism in cancer stem cells.Biochem Pharmacol. 2023 Jun;212:115550. doi: 10.1016/j.bcp.2023.115550. Epub 2023 Apr 13. Biochem Pharmacol. 2023. PMID: 37060962 Review.
Cited by
-
Effects of the Combined Treatment with a G-Quadruplex-Stabilizing Ligand and Photon Beams on Glioblastoma Stem-like Cells: A Magnetic Resonance Study.Int J Mol Sci. 2021 Nov 24;22(23):12709. doi: 10.3390/ijms222312709. Int J Mol Sci. 2021. PMID: 34884511 Free PMC article.
-
Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism.Biomedicines. 2023 May 5;11(5):1365. doi: 10.3390/biomedicines11051365. Biomedicines. 2023. PMID: 37239036 Free PMC article. Review.
-
Metabolic Reprogramming in Cancer Cells: Emerging Molecular Mechanisms and Novel Therapeutic Approaches.Pharmaceutics. 2022 Jun 19;14(6):1303. doi: 10.3390/pharmaceutics14061303. Pharmaceutics. 2022. PMID: 35745875 Free PMC article. Review.
-
Mechanism of cancer stemness maintenance in human liver cancer.Cell Death Dis. 2022 Apr 21;13(4):394. doi: 10.1038/s41419-022-04848-z. Cell Death Dis. 2022. PMID: 35449193 Free PMC article. Review.
-
Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases.Cells. 2025 Mar 29;14(7):511. doi: 10.3390/cells14070511. Cells. 2025. PMID: 40214466 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

