Effect of Apixaban Pretreatment on Alteplase-Induced Thrombolysis: An In Vitro Study
- PMID: 34603054
- PMCID: PMC8479181
- DOI: 10.3389/fphar.2021.740930
Effect of Apixaban Pretreatment on Alteplase-Induced Thrombolysis: An In Vitro Study
Abstract
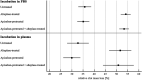
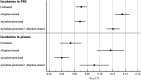
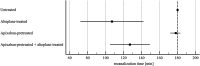
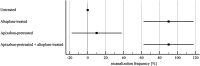
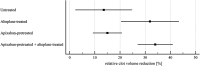
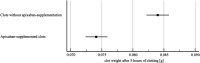
Benefit of thrombolytic therapy in patients with acute stroke, who are on anticoagulant treatment, is not well addressed. The aim of this study was to investigate whether apixaban can modify the thrombolytic efficacy of alteplase in vitro. Static and flow models and two variants of red blood cell (RBC) dominant clots, with and without apixaban, were used. Clots were prepared from the blood of healthy human donors and subsequently exposed to alteplase treatment. Apixaban and alteplase were used in clinically relevant concentrations. Clot lysis in the static model was determined both by clot weight and spectrophotometric determination of RBC release. Clot lysis in the flow model was determined by measuring recanalization time, clot length and spectrophotometric determination of RBC release. In the static model, clots without apixaban; compared to those with apixaban had alteplase-induced mass loss 54 ± 8% vs. 53 ± 8%, p = 1.00; RBC release 0.14 ± 0.04 vs. 0.12 ± 0.04, p = 0.14, respectively. Very similar results were obtained if plasma was used instead of physiological buffered saline as the incubation medium. In the flow model, clot lysis without apixaban; compared to those with apixaban was as follows: recanalization time 107 ± 46 min vs. 127 ± 31 min, p = 1.00; recanalization frequency 90 ± 22% vs. 90 ± 22%, p = 1.00; clot volume reduction 32 ± 15% vs. 34 ± 10%, p = 1.00; RBC release 0.029 ± 0.007 vs. 0.022 ± 0.007, p = 0.16, respectively. Apixaban had no positive effect on alteplase-induced thrombolysis in both the in vitro static and flow models. Our data support current clinical practice, such that thrombolysis is contraindicated in stroke treatment for patients who have been treated with anticoagulants.
Keywords: alteplase; apixaban; clot; in vitro; stroke; thrombolysis.
Copyright © 2021 Thalerová, Pešková, Kittová, Gulati, Víteček, Kubala and Mikulík.
Conflict of interest statement
This study received funding from the Pfizer Inc. through a competitive grant from the BMS/Pfizer European Thrombosis Investigator Initiated Research Program (ERISTA). The funder was not involved in the study design, collection, analysis, interpretation of data and the writing of this article. The submission for publication was approved by the funder. All authors declare no other competing interests.
Figures

Similar articles
-
A collateral circulation in ischemic stroke accelerates recanalization due to lower clot compaction.PLoS One. 2024 Nov 19;19(11):e0314079. doi: 10.1371/journal.pone.0314079. eCollection 2024. PLoS One. 2024. PMID: 39561145 Free PMC article.
-
Clot penetration and fibrin binding of amediplase,a chimeric plasminogen activator (K2 tu-PA).Thromb Haemost. 2004 Jan;91(1):52-60. doi: 10.1160/TH03-07-0435. Thromb Haemost. 2004. PMID: 14691568
-
3K3A-Activated Protein C Variant Does Not Interfere With the Plasma Clot Lysis Activity of Tenecteplase.Stroke. 2020 Jul;51(7):2236-2239. doi: 10.1161/STROKEAHA.120.028793. Epub 2020 Jun 17. Stroke. 2020. PMID: 32568648 Free PMC article.
-
Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data.Lancet. 2019 Jul 13;394(10193):139-147. doi: 10.1016/S0140-6736(19)31053-0. Epub 2019 May 22. Lancet. 2019. PMID: 31128925
-
Recanalization therapies for acute ischemic stroke.Semin Neurol. 1998;18(4):471-84. doi: 10.1055/s-2008-1040900. Semin Neurol. 1998. PMID: 9932618 Review.
Cited by
-
Computer-aided engineering of staphylokinase toward enhanced affinity and selectivity for plasmin.Comput Struct Biotechnol J. 2022 Mar 12;20:1366-1377. doi: 10.1016/j.csbj.2022.03.004. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35386102 Free PMC article.
-
A collateral circulation in ischemic stroke accelerates recanalization due to lower clot compaction.PLoS One. 2024 Nov 19;19(11):e0314079. doi: 10.1371/journal.pone.0314079. eCollection 2024. PLoS One. 2024. PMID: 39561145 Free PMC article.
-
Factors influencing the efficacy of recombinant tissue plasminogen activator: Implications for ischemic stroke treatment.PLoS One. 2024 Jun 6;19(6):e0302269. doi: 10.1371/journal.pone.0302269. eCollection 2024. PLoS One. 2024. PMID: 38843177 Free PMC article.
References
LinkOut - more resources
Full Text Sources

