Combinations of Single Chain Variable Fragments From HIV Broadly Neutralizing Antibodies Demonstrate High Potency and Breadth
- PMID: 34603312
- PMCID: PMC8481832
- DOI: 10.3389/fimmu.2021.734110
Combinations of Single Chain Variable Fragments From HIV Broadly Neutralizing Antibodies Demonstrate High Potency and Breadth
Abstract
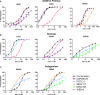
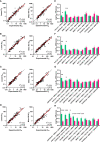
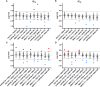
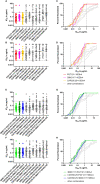
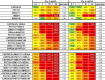
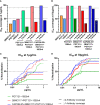
Broadly neutralizing antibodies (bNAbs) are currently being assessed in clinical trials for their ability to prevent HIV infection. Single chain variable fragments (scFv) of bNAbs have advantages over full antibodies as their smaller size permits improved diffusion into mucosal tissues and facilitates vector-driven gene expression. We have previously shown that scFv of bNAbs individually retain significant breadth and potency. Here we tested combinations of five scFv derived from bNAbs CAP256-VRC26.25 (V2-apex), PGT121 (N332-supersite), 3BNC117 (CD4bs), 8ANC195 (gp120-gp41 interface) and 10E8v4 (MPER). Either two or three scFv were combined in equimolar amounts and tested in the TZM-bl neutralization assay against a multiclade panel of 17 viruses. Experimental IC50 and IC80 data were compared to predicted neutralization titers based on single scFv titers using the Loewe additive and the Bliss-Hill model. Like full-sized antibodies, combinations of scFv showed significantly improved potency and breadth compared to single scFv. Combinations of two or three scFv generally followed an independent action model for breadth and potency with no significant synergy or antagonism observed overall although some exceptions were noted. The Loewe model underestimated potency for some dual and triple combinations while the Bliss-Hill model was better at predicting IC80 titers of triple combinations. Given this, we used the Bliss-Hill model to predict the coverage of scFv against a 45-virus panel at concentrations that correlated with protection in the AMP trials. Using IC80 titers and concentrations of 1μg/mL, there was 93% coverage for one dual scFv combination (3BNC117+10E8v4), and 96% coverage for two of the triple combinations (CAP256.25+3BNC117+10E8v4 and PGT121+3BNC117+10E8v4). Combinations of scFv, therefore, show significantly improved breadth and potency over individual scFv and given their size advantage, have potential for use in passive immunization.
Keywords: HIV; HIV prevention; broadly neutralizing antibodies; combinations of scFv; single chain variable fragments.
Copyright © 2021 van Dorsten, Wagh, Moore and Morris.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Neutralization Breadth and Potency of Single-Chain Variable Fragments Derived from Broadly Neutralizing Antibodies Targeting Multiple Epitopes on the HIV-1 Envelope.J Virol. 2020 Jan 6;94(2):e01533-19. doi: 10.1128/JVI.01533-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31619559 Free PMC article.
-
Single-Chain Variable Fragments of Broadly Neutralizing Antibodies Prevent HIV Cell-Cell Transmission.J Virol. 2022 Feb 23;96(4):e0193421. doi: 10.1128/jvi.01934-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935437 Free PMC article.
-
HIV Broadly Neutralizing Antibodies Expressed as IgG3 Preserve Neutralization Potency and Show Improved Fc Effector Function.Front Immunol. 2021 Sep 10;12:733958. doi: 10.3389/fimmu.2021.733958. eCollection 2021. Front Immunol. 2021. PMID: 34566999 Free PMC article.
-
Broadly Neutralizing Antibodies for HIV Prevention.Annu Rev Med. 2020 Jan 27;71:329-346. doi: 10.1146/annurev-med-110118-045506. Annu Rev Med. 2020. PMID: 31986089 Review.
-
Clinical Trials of Broadly Neutralizing Monoclonal Antibodies for Human Immunodeficiency Virus Prevention: A Review.J Infect Dis. 2021 Feb 13;223(3):370-380. doi: 10.1093/infdis/jiaa377. J Infect Dis. 2021. PMID: 32604408 Free PMC article. Review.
Cited by
-
Adenovirus vector-mediated single chain variable fragments target the nucleocapsid protein of porcine epidemic diarrhea virus and protect against viral infection in piglets.Front Immunol. 2023 Jan 24;14:1058327. doi: 10.3389/fimmu.2023.1058327. eCollection 2023. Front Immunol. 2023. PMID: 36761768 Free PMC article.
-
An oncolytic herpes simplex virus type 1 strain expressing a single-chain variable region antibody fragment against PD-1 and a PI3K inhibitor synergize to elicit antitumor immunity in ovarian cancer.Arch Virol. 2023 Mar 31;168(4):128. doi: 10.1007/s00705-023-05754-1. Arch Virol. 2023. PMID: 37002434
-
Broadly neutralizing antibodies for HIV prevention: a comprehensive review and future perspectives.Clin Microbiol Rev. 2024 Jun 13;37(2):e0015222. doi: 10.1128/cmr.00152-22. Epub 2024 Apr 30. Clin Microbiol Rev. 2024. PMID: 38687039 Free PMC article. Review.
-
A comprehensive engineering strategy improves potency and manufacturability of a near pan-neutralizing antibody against HIV.Structure. 2025 Jul 3;33(7):1150-1164.e8. doi: 10.1016/j.str.2025.04.016. Epub 2025 May 14. Structure. 2025. PMID: 40373766 Free PMC article.
-
HIV-1 bispecific antibody iMab-N6 exhibits enhanced breadth but not potency over its parental antibodies iMab and N6.Virol J. 2022 Sep 7;19(1):143. doi: 10.1186/s12985-022-01876-1. Virol J. 2022. PMID: 36071449 Free PMC article.
References
-
- Saunders KO, Wang L, Joyce MG, Yang Z-YY, Balazs AB, Cheng C, et al. Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates From Mucosal Simian-Human Immunodeficiency Virus Infection. J Virol (2015) 89:8334–45. doi: 10.1128/jvi.00908-15 - DOI - PMC - PubMed
-
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, et al. Antibody Protects Macaques Against Vaginal Challenge With a Pathogenic R5 Simian/Human Immunodeficiency Virus at Serum Levels Giving Complete Neutralization In Vitro . J Virol (2001) 75:8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

