Contribution to HIV Prevention and Treatment by Antibody-Mediated Effector Function and Advances in Broadly Neutralizing Antibody Delivery by Vectored Immunoprophylaxis
- PMID: 34603314
- PMCID: PMC8479175
- DOI: 10.3389/fimmu.2021.734304
Contribution to HIV Prevention and Treatment by Antibody-Mediated Effector Function and Advances in Broadly Neutralizing Antibody Delivery by Vectored Immunoprophylaxis
Abstract
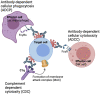
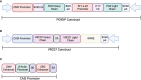
HIV-1 broadly neutralizing antibodies (bNAbs) targeting the viral envelope have shown significant promise in both HIV prevention and viral clearance, including pivotal results against sensitive strains in the recent Antibody Mediated Prevention (AMP) trial. Studies of bNAb passive transfer in infected patients have demonstrated transient reduction of viral load at high concentrations that rebounds as bNAb is cleared from circulation. While neutralization is a crucial component of therapeutic efficacy, numerous studies have demonstrated that bNAbs can also mediate effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent complement deposition (ADCD). These functions have been shown to contribute towards protection in several models of HIV acquisition and in viral clearance during chronic infection, however the role of target epitope in facilitating these functions, as well as the contribution of individual innate functions in protection and viral clearance remain areas of active investigation. Despite their potential, the transient nature of antibody passive transfer limits the widespread use of bNAbs. To overcome this, we and others have demonstrated vectored antibody delivery capable of yielding long-lasting expression of bNAbs in vivo. Two clinical trials have shown that adeno-associated virus (AAV) delivery of bNAbs is safe and capable of sustained bNAb expression for over 18 months following a single intramuscular administration. Here, we review key concepts of effector functions mediated by bNAbs against HIV infection and the potential for vectored immunoprophylaxis as a means of producing bNAbs in patients.
Keywords: AAV; Fc receptor; HIV; VRC07; broadly neutralizing antibody; humanized mice; innate immunity; vectored immunoprophylaxis.
Copyright © 2021 Phelps and Balazs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. . The Neutralization Breadth of HIV-1 Develops Incrementally Over Four Years and Is Associated With CD4+ T Cell Decline and High Viral Load During Acute Infection. J Virol (2011) 85(10):4828–40. doi: 10.1128/JVI.00198-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

