Stimulus-Specific Expression, Selective Generation and Novel Function of Grass Carp (Ctenopharyngodon idella) IL-12 Isoforms: New Insights Into the Heterodimeric Cytokines in Teleosts
- PMID: 34603315
- PMCID: PMC8481787
- DOI: 10.3389/fimmu.2021.734535
Stimulus-Specific Expression, Selective Generation and Novel Function of Grass Carp (Ctenopharyngodon idella) IL-12 Isoforms: New Insights Into the Heterodimeric Cytokines in Teleosts
Abstract
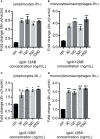
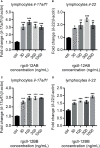
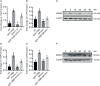
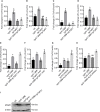
Interleukin-12 (IL-12) is a heterodimeric cytokine composed of a p35 subunit specific to IL-12 and a p40 subunit shared with IL-23. In this study, we unveiled the existence of two p35 paralogues in grass carp (named gcp35a and gcp35b). Notably, gcp35a and gcp35b displayed distinct inducible expression patterns, as poly I:C merely induced the gene expression of gcp35a but not gcp35b, while recombinant grass carp interferon-gamma (rgcIfn-γ) only enhanced the transcription of gcp35b but not gcp35a. Moreover, the signaling mechanisms responsible for the inducible expression of gcp35a and gcp35b mRNA were elucidated. Because of the existence of three grass carp p40 genes (gcp40a, gcp40b and gcp40c) and two p35 paralogues, six gcIl-12 isoforms were predicted by 3D modeling. Results showed that gcp40a and gcp40b but not gcp40c had the potential for forming heterodimers with both gcp35 paralogues via the disulfide bonds. Non-reducing electrophoresis experiments further disclosed that only gcp40b but not gcp40a or gcp40c could form heterodimers with gcp35 to produce secretory heterodimeric gcp35a/gcp40b (gcIl-12AB) and gcp35b/gcp40b (gcIl-12BB), which prompted us to prepare their recombinant proteins. These two recombinant proteins exhibited their extensive regulation on Ifn-γ production in various immune cells. Intriguingly, both gcIl-12 isoforms significantly enhanced the transcription of il-17a/f1 and il-22 in lymphocytes, and their regulation on il-17a/f1 expression was mediated by Stat3/Rorγt signaling, supporting the potential of gcIl-12 isoforms for inducing Th17-like responses. Additionally, stimulatory effects of gcIl-12 isoforms on il-17a/f1 and ifn-γ expression were attenuated by gcTgf-β1 via suppressing the activation of Stat3 signaling, implying that their signaling could be manipulated. In brief, our works provide new insights into the inducible expression pattern, heterodimeric generation and functional novelty of Il-12 isoforms in teleosts.
Keywords: Il-12; Th17-like response; grass carp; heterodimeric form; p35 paralogues.
Copyright © 2021 Qiu, Sun, Wang, Ren, Wang, Zhang, Yang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Novel functions of grass carp three p40 isoforms as modulators of Th17 signature cytokine expression in head kidney leukocytes.Fish Shellfish Immunol. 2020 Mar;98:995-1000. doi: 10.1016/j.fsi.2019.11.025. Epub 2019 Nov 14. Fish Shellfish Immunol. 2020. PMID: 31734285
-
Functional characterization of three fish-specific interleukin-23 isoforms as regulators of Th17 signature cytokine expression in grass carp head kidney leukocytes.Fish Shellfish Immunol. 2019 Sep;92:315-321. doi: 10.1016/j.fsi.2019.06.028. Epub 2019 Jun 13. Fish Shellfish Immunol. 2019. PMID: 31202965
-
Identification of a single p19 gene and three p40 paralogues in grass carp (Ctenopharyngodon idellus): Their potential for the formation of interleukin 23 and inducible expression in vitro and in vivo.Fish Shellfish Immunol. 2017 Dec;71:434-442. doi: 10.1016/j.fsi.2017.10.009. Epub 2017 Oct 10. Fish Shellfish Immunol. 2017. PMID: 29024769
-
The IL-12 family cytokines in fish: Molecular structure, expression profile and function.Dev Comp Immunol. 2023 Apr;141:104643. doi: 10.1016/j.dci.2023.104643. Epub 2023 Jan 9. Dev Comp Immunol. 2023. PMID: 36632929 Review.
-
Biogenesis and engineering of interleukin 12 family cytokines.Trends Biochem Sci. 2022 Nov;47(11):936-949. doi: 10.1016/j.tibs.2022.05.005. Epub 2022 Jun 10. Trends Biochem Sci. 2022. PMID: 35691784 Review.
References
-
- Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, et al. . Cloning of cDNA for Natural Killer Cell Stimulatory Factor, a Heterodimeric Cytokine With Multiple Biologic Effects on T and Natural Killer Cells. J Immunol (1991) 146(9):3074–81. - PubMed
-
- Babik JM, Adams E, Tone Y, Fairchild PJ, Tone M, Waldmann H. Expression of Murine IL-12 is Regulated by Translational Control of the P35 Subunit. J Immunol (1999) 162(7):4069–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

