A Hypoxia Signature for Predicting Prognosis and Tumor Immune Microenvironment in Adrenocortical Carcinoma
- PMID: 34603443
- PMCID: PMC8481041
- DOI: 10.1155/2021/2298973
A Hypoxia Signature for Predicting Prognosis and Tumor Immune Microenvironment in Adrenocortical Carcinoma
Abstract
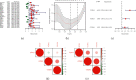
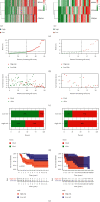
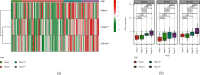
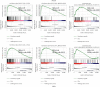
Adrenocortical carcinoma (ACC) is a rare malignancy with dismal prognosis. Hypoxia is one of characteristics of cancer leading to tumor progression. For ACC, however, no reliable prognostic signature on the basis of hypoxia genes has been built. Our study aimed to develop a hypoxia-associated gene signature in ACC. Data of ACC patients were obtained from TCGA and GEO databases. The genes included in hypoxia risk signature were identified using the Cox regression analysis as well as LASSO regression analysis. GSEA was applied to discover the enriched gene sets. To detect a possible connection between the gene signature and immune cells, the CIBERSORT technique was applied. In ACC, the hypoxia signature including three genes (CCNA2, COL5A1, and EFNA3) was built to predict prognosis and reflect the immune microenvironment. Patients with high-risk scores tended to have a poor prognosis. According to the multivariate regression analysis, the hypoxia signature could be served as an independent indicator in ACC patients. GSEA demonstrated that gene sets linked to cancer proliferation and cell cycle were differentially enriched in high-risk classes. Additionally, we found that PDL1 and CTLA4 expression were significantly lower in the high-risk group than in the low-risk group, and resting NK cells displayed a significant increase in the high-risk group. In summary, the hypoxia risk signature created in our study might predict prognosis and evaluate the tumor immune microenvironment for ACC.
Copyright © 2021 Xi Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Identification of a Ferroptosis-Related Signature Associated with Prognosis and Immune Infiltration in Adrenocortical Carcinoma.Int J Endocrinol. 2021 Jul 20;2021:4654302. doi: 10.1155/2021/4654302. eCollection 2021. Int J Endocrinol. 2021. PMID: 34335745 Free PMC article.
-
Development and Validation of a Hypoxia-Related Signature for Predicting Survival Outcomes in Patients With Bladder Cancer.Front Genet. 2021 May 26;12:670384. doi: 10.3389/fgene.2021.670384. eCollection 2021. Front Genet. 2021. PMID: 34122523 Free PMC article.
-
Identification of a chromatin regulator signature and potential prognostic ability for adrenocortical carcinoma.Front Genet. 2022 Aug 26;13:948353. doi: 10.3389/fgene.2022.948353. eCollection 2022. Front Genet. 2022. PMID: 36092868 Free PMC article.
-
Analysis of m6A-Related Signatures in the Tumor Immune Microenvironment and Identification of Clinical Prognostic Regulators in Adrenocortical Carcinoma.Front Immunol. 2021 Mar 3;12:637933. doi: 10.3389/fimmu.2021.637933. eCollection 2021. Front Immunol. 2021. PMID: 33746977 Free PMC article.
-
Construction of a risk signature for adrenocortical carcinoma using immune-related genes.Transl Androl Urol. 2020 Oct;9(5):1920-1930. doi: 10.21037/tau-20-485. Transl Androl Urol. 2020. PMID: 33209656 Free PMC article.
Cited by
-
Hypoxia signaling pathway: A central mediator in endocrine tumors.Front Endocrinol (Lausanne). 2023 Jan 9;13:1103075. doi: 10.3389/fendo.2022.1103075. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36699028 Free PMC article. Review.
-
FBP1 is a potential prognostic biomarker and correlated with tumor immunosuppressive microenvironment in glioblastoma.Neurosurg Rev. 2023 Jul 29;46(1):187. doi: 10.1007/s10143-023-02097-y. Neurosurg Rev. 2023. PMID: 37507483
-
The Effect of Hypoxia and Hypoxia-Associated Pathways in the Regulation of Antitumor Response: Friends or Foes?Front Immunol. 2022 Feb 8;13:828875. doi: 10.3389/fimmu.2022.828875. eCollection 2022. Front Immunol. 2022. PMID: 35211123 Free PMC article. Review.
-
Construction and validation of a novel prognostic model with palmitoylation-related genes for glioblastoma.Transl Cancer Res. 2024 Nov 30;13(11):6117-6135. doi: 10.21037/tcr-24-787. Epub 2024 Nov 27. Transl Cancer Res. 2024. PMID: 39697698 Free PMC article.
-
A Novel Predictive Model for Adrenocortical Carcinoma Based on Hypoxia- and Ferroptosis-Related Gene Expression.Front Med (Lausanne). 2022 May 16;9:856606. doi: 10.3389/fmed.2022.856606. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35652069 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

