Effect of ORF7 of SARS-CoV-2 on the Chemotaxis of Monocytes and Neutrophils In Vitro
- PMID: 34603560
- PMCID: PMC8483903
- DOI: 10.1155/2021/6803510
Effect of ORF7 of SARS-CoV-2 on the Chemotaxis of Monocytes and Neutrophils In Vitro
Abstract
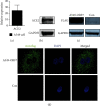
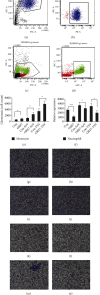
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently the most significant public health threat worldwide. Patients with severe COVID-19 usually have pneumonia concomitant with local inflammation and sometimes a cytokine storm. Specific components of the SARS-CoV-2 virus trigger lung inflammation, and recruitment of immune cells to the lungs exacerbates this process, although much remains unknown about the pathogenesis of COVID-19. Our study of lung type II pneumocyte cells (A549) demonstrated that ORF7, an open reading frame (ORF) in the genome of SARS-CoV-2, induced the production of CCL2, a chemokine that promotes the chemotaxis of monocytes, and decreased the expression of IL-8, a chemokine that recruits neutrophils. A549 cells also had an increased level of IL-6. The results of our chemotaxis Transwell assay suggested that ORF7 augmented monocyte infiltration and reduced the number of neutrophils. We conclude that the ORF7 of SARS-CoV-2 may have specific effects on the immunological changes in tissues after infection. These results suggest that the functions of other ORFs of SARS-CoV-2 should also be comprehensively examined.
Copyright © 2021 Gang Wang et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Long-term infection of SARS-CoV-2 changed the body's immune status.Clin Immunol. 2020 Sep;218:108524. doi: 10.1016/j.clim.2020.108524. Epub 2020 Jul 11. Clin Immunol. 2020. PMID: 32659373 Free PMC article.
-
Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro.J Virol. 2006 Mar;80(6):2684-93. doi: 10.1128/JVI.80.6.2684-2693.2006. J Virol. 2006. PMID: 16501078 Free PMC article.
-
Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19.J Biol Regul Homeost Agents. 2020 Sep-Oct,;34(5):1629-1632. doi: 10.23812/20-2EDIT. J Biol Regul Homeost Agents. 2020. PMID: 32945158
-
Role of CCL2/CCR2 axis in the pathogenesis of COVID-19 and possible Treatments: All options on the Table.Int Immunopharmacol. 2022 Dec;113(Pt A):109325. doi: 10.1016/j.intimp.2022.109325. Epub 2022 Oct 14. Int Immunopharmacol. 2022. PMID: 36252475 Free PMC article. Review.
-
Unpacking Pandora From Its Box: Deciphering the Molecular Basis of the SARS-CoV-2 Coronavirus.Int J Mol Sci. 2020 Dec 31;22(1):386. doi: 10.3390/ijms22010386. Int J Mol Sci. 2020. PMID: 33396557 Free PMC article. Review.
Cited by
-
Characterization of humoral immune responses against SARS-CoV-2 accessory proteins in infected patients and mouse model.Virol Sin. 2024 Jun;39(3):414-421. doi: 10.1016/j.virs.2024.04.005. Epub 2024 Apr 26. Virol Sin. 2024. PMID: 38677713 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

