One-Two Punch Therapy for the Treatment of T-Cell Malignancies Involving p53-Dependent Cellular Senescence
- PMID: 34603598
- PMCID: PMC8481056
- DOI: 10.1155/2021/5529518
One-Two Punch Therapy for the Treatment of T-Cell Malignancies Involving p53-Dependent Cellular Senescence
Abstract
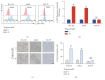
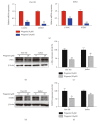
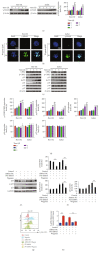
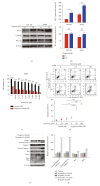
T-cell malignancies are still difficult to treat due to a paucity of plans that target critical dependencies. Drug-induced cellular senescence provides a permanent cell cycle arrest during tumorigenesis and cancer development, particularly when combined with senolytics to promote apoptosis of senescent cells, which is an innovation for cancer therapy. Here, our research found that wogonin, a well-known natural flavonoid compound, not only had a potential to inhibit cell growth and proliferation but also induced cellular senescence in T-cell malignancies with nonlethal concentration. Transcription activity of senescence-suppression human telomerase reverse transcriptase (hTERT) and oncogenic C-MYC was suppressed in wogonin-induced senescent cells, resulting in the inhibition of telomerase activity. We also substantiated the occurrence of DNA damage during the wogonin-induced aging process. Results showed that wogonin increased the activity of senescence-associated β-galactosidase (SA-β-Gal) and activated the DNA damage response pathway mediated by p53. In addition, we found the upregulated expression of BCL-2 in senescent T-cell malignancies because of the antiapoptotic properties of senescent cells. Following up this result, we identified a BCL-2 inhibitor Navitoclax (ABT-263), which was highly effective in decreasing cell viability and inducing apoptotic cell death in wogonin-induced senescent cells. Thus, the "one-two punch" approach increased the sensibility of T-cell malignancies with low expression of BCL-2 to Navitoclax. In conclusion, our research revealed that wogonin possesses potential antitumor effects based on senescence induction, offering a better insight into the development of novel therapeutic methods for T-cell malignancies.
Copyright © 2021 Yingjie Qing et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Uncovering cellular senescence as a therapeutic target in NF2-related vestibular schwannoma.Hear Res. 2025 Jan;455:109165. doi: 10.1016/j.heares.2024.109165. Epub 2024 Dec 4. Hear Res. 2025. PMID: 39647233
-
Mitotic catastrophe and p53-dependent senescence induction in T-cell malignancies exposed to nonlethal dosage of GL-V9.Arch Toxicol. 2020 Jan;94(1):305-323. doi: 10.1007/s00204-019-02623-2. Epub 2019 Nov 23. Arch Toxicol. 2020. PMID: 31760435
-
Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity.Aging Cell. 2020 Apr;19(4):e13142. doi: 10.1111/acel.13142. Epub 2020 Mar 31. Aging Cell. 2020. PMID: 32233024 Free PMC article.
-
Role of p53 in the Regulation of Cellular Senescence.Biomolecules. 2020 Mar 8;10(3):420. doi: 10.3390/biom10030420. Biomolecules. 2020. PMID: 32182711 Free PMC article. Review.
-
Senotherapeutics for HIV and aging.Curr Opin HIV AIDS. 2020 Mar;15(2):83-93. doi: 10.1097/COH.0000000000000609. Curr Opin HIV AIDS. 2020. PMID: 31833962 Free PMC article. Review.
Cited by
-
Involvement of Bcl-2 Family Proteins in Tetraploidization-Related Senescence.Int J Mol Sci. 2023 Mar 28;24(7):6374. doi: 10.3390/ijms24076374. Int J Mol Sci. 2023. PMID: 37047342 Free PMC article. Review.
-
Senescent T Cells in Age-Related Diseases.Aging Dis. 2024 Mar 8;16(1):321-44. doi: 10.14336/AD.2024.0219. Online ahead of print. Aging Dis. 2024. PMID: 38502582 Free PMC article. Review.
-
Multiple characteristic alterations and available therapeutic strategies of cellular senescence.J Zhejiang Univ Sci B. 2023 Feb 15;24(2):101-114. doi: 10.1631/jzus.B2200178. J Zhejiang Univ Sci B. 2023. PMID: 36751697 Free PMC article. Review.
-
A Conversation with ChatGPT on Contentious Issues in Senescence and Cancer Research.Mol Pharmacol. 2024 Apr 17;105(5):313-327. doi: 10.1124/molpharm.124.000871. Mol Pharmacol. 2024. PMID: 38458774 Free PMC article.
-
The Potential of Senescence as a Target for Developing Anticancer Therapy.Int J Mol Sci. 2023 Feb 8;24(4):3436. doi: 10.3390/ijms24043436. Int J Mol Sci. 2023. PMID: 36834846 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

