Hydrogen Sulfide Attenuates Angiotensin II-Induced Cardiac Fibroblast Proliferation and Transverse Aortic Constriction-Induced Myocardial Fibrosis through Oxidative Stress Inhibition via Sirtuin 3
- PMID: 34603602
- PMCID: PMC8486544
- DOI: 10.1155/2021/9925771
Hydrogen Sulfide Attenuates Angiotensin II-Induced Cardiac Fibroblast Proliferation and Transverse Aortic Constriction-Induced Myocardial Fibrosis through Oxidative Stress Inhibition via Sirtuin 3
Abstract
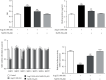
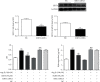
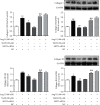
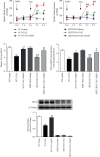
Sirtuin 3 (SIRT3) is critical in mitochondrial function and oxidative stress. Our present study investigates whether hydrogen sulfide (H2S) attenuated myocardial fibrosis and explores the possible role of SIRT3 on the protective effects. Neonatal rat cardiac fibroblasts were pretreated with NaHS followed by angiotensin II (Ang II) stimulation. SIRT3 was knocked down with siRNA technology. SIRT3 promoter activity and expression, as well as mitochondrial function, were measured. Male wild-type (WT) and SIRT3 knockout (KO) mice were intraperitoneally injected with NaHS followed by transverse aortic constriction (TAC). Myocardium sections were stained with Sirius red. Hydroxyproline content, collagen I and collagen III, α-smooth muscle actin (α-SMA), and dynamin-related protein 1 (DRP1) expression were measured both in vitro and in vivo. We found that NaHS enhanced SIRT3 promoter activity and increased SIRT3 mRNA expression. NaHS inhibited cell proliferation and hydroxyproline secretion, decreased collagen I, collagen III, α-SMA, and DRP1 expression, alleviated oxidative stress, and improved mitochondrial respiration function and membrane potential in Ang II-stimulated cardiac fibroblasts, which were unavailable after SIRT3 was silenced. In vivo, NaHS reduced hydroxyproline content, ameliorated perivascular and interstitial collagen deposition, and inhibited collagen I, collagen III, and DRP1 expression in the myocardium of WT mice but not SIRT3 KO mice with TAC. Altogether, NaHS attenuated myocardial fibrosis through oxidative stress inhibition via a SIRT3-dependent manner.
Copyright © 2021 Lulu Liu et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures




Similar articles
-
Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner.Br J Pharmacol. 2018 Apr;175(8):1126-1145. doi: 10.1111/bph.13861. Epub 2017 Jul 6. Br J Pharmacol. 2018. PMID: 28503736 Free PMC article.
-
Necroptosis Inhibition by Hydrogen Sulfide Alleviated Hypoxia-Induced Cardiac Fibroblasts Proliferation via Sirtuin 3.Int J Mol Sci. 2021 Nov 2;22(21):11893. doi: 10.3390/ijms222111893. Int J Mol Sci. 2021. PMID: 34769322 Free PMC article.
-
Exogenous Hydrogen Sulfide Supplement Attenuates Isoproterenol-Induced Myocardial Hypertrophy in a Sirtuin 3-Dependent Manner.Oxid Med Cell Longev. 2018 Dec 17;2018:9396089. doi: 10.1155/2018/9396089. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30647820 Free PMC article.
-
SIRT3 sulfhydration using hydrogen sulfide inhibited angiotensin II-induced atrial fibrosis and vulnerability to atrial fibrillation via suppression of the TGF-β1/smad2/3 signalling pathway.Eur J Pharmacol. 2024 Nov 5;982:176900. doi: 10.1016/j.ejphar.2024.176900. Epub 2024 Aug 19. Eur J Pharmacol. 2024. PMID: 39168432
-
Hydrogen Sulfide Donor GYY4137 Protects against Myocardial Fibrosis.Oxid Med Cell Longev. 2015;2015:691070. doi: 10.1155/2015/691070. Epub 2015 May 11. Oxid Med Cell Longev. 2015. PMID: 26078813 Free PMC article.
Cited by
-
MicroRNA-499-5p inhibits transforming growth factor-β1-induced Smad2 signaling pathway and suppresses fibroblast proliferation and collagen synthesis in rat by targeting TGFβ-R1.Mol Biol Rep. 2023 Dec;50(12):9757-9767. doi: 10.1007/s11033-023-08755-0. Epub 2023 Sep 7. Mol Biol Rep. 2023. PMID: 37676431 Free PMC article.
-
Sirtuins as Endogenous Regulators of Cardiac Fibrosis: A Current Perspective.Cardiovasc Toxicol. 2025 Aug 11. doi: 10.1007/s12012-025-10052-0. Online ahead of print. Cardiovasc Toxicol. 2025. PMID: 40789811
-
Hydroxysafflor yellow A mitigates myocardial fibrosis induced by isoproterenol and angiotensin II.Am J Transl Res. 2022 Dec 15;14(12):8588-8598. eCollection 2022. Am J Transl Res. 2022. PMID: 36628216 Free PMC article.
-
Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1.Open Life Sci. 2023 Feb 9;18(1):20220554. doi: 10.1515/biol-2022-0554. eCollection 2023. Open Life Sci. 2023. PMID: 36816804 Free PMC article.
-
H2S Protects Against Immobilization-Induced Muscle Atrophy via Reducing Oxidative Stress and Inflammation.Front Physiol. 2022 Apr 6;13:844539. doi: 10.3389/fphys.2022.844539. eCollection 2022. Front Physiol. 2022. PMID: 35464091 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

