Recent progress and future challenges in the supramolecular polymerization of metal-containing monomers
- PMID: 34603655
- PMCID: PMC8480320
- DOI: 10.1039/d1sc03388c
Recent progress and future challenges in the supramolecular polymerization of metal-containing monomers
Abstract
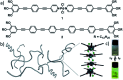
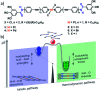
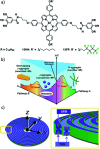
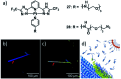
The self-assembly of discrete molecular entities into functional nanomaterials has become a major research area in the past decades. The library of investigated compounds has diversified significantly, while the field as a whole has matured. The incorporation of metal ions in the molecular design of the (supra-)molecular building blocks greatly expands the potential applications, while also offering a promising approach to control molecular recognition and attractive and/or repulsive intermolecular binding events. Hence, supramolecular polymerization of metal-containing monomers has emerged as a major research focus in the field. In this perspective article, we highlight recent significant advances in supramolecular polymerization of metal-containing monomers and discuss their implications for future research. Additionally, we also outline some major challenges that metallosupramolecular chemists (will) have to face to produce metallosupramolecular polymers (MSPs) with advanced applications and functionalities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


















Similar articles
-
Controllable coordination-driven self-assembly: from discrete metallocages to infinite cage-based frameworks.Acc Chem Res. 2015 Feb 17;48(2):201-10. doi: 10.1021/ar5003076. Epub 2014 Dec 17. Acc Chem Res. 2015. PMID: 25517043
-
Macrocycle-Based Solid-State Supramolecular Polymers.Acc Chem Res. 2022 Apr 5;55(7):1025-1034. doi: 10.1021/acs.accounts.2c00011. Epub 2022 Mar 24. Acc Chem Res. 2022. PMID: 35321546
-
Metallosupramolecular Architectures Obtained from Poly-N-heterocyclic Carbene Ligands.Acc Chem Res. 2017 Sep 19;50(9):2167-2184. doi: 10.1021/acs.accounts.7b00158. Epub 2017 Aug 25. Acc Chem Res. 2017. PMID: 28841284
-
When polymerization meets coordination-driven self-assembly: metallo-supramolecular polymers based on supramolecular coordination complexes.Chem Soc Rev. 2021 Jul 7;50(13):7395-7417. doi: 10.1039/d0cs00654h. Epub 2021 May 21. Chem Soc Rev. 2021. PMID: 34018496 Review.
-
Metallosupramolecular polymers: current status and future prospects.Chem Soc Rev. 2023 Dec 11;52(24):8635-8650. doi: 10.1039/d3cs00692a. Chem Soc Rev. 2023. PMID: 37962512 Review.
Cited by
-
Anti-cooperative Self-Assembly with Maintained Emission Regulated by Conformational and Steric Effects.Angew Chem Int Ed Engl. 2022 Apr 19;61(17):e202200390. doi: 10.1002/anie.202200390. Epub 2022 Mar 2. Angew Chem Int Ed Engl. 2022. PMID: 35112463 Free PMC article.
-
Multi-chiral materials comprising metallosupramolecular and covalent helical polymers containing five axial motifs within a helix.Nat Commun. 2023 Jun 8;14(1):3348. doi: 10.1038/s41467-023-39014-2. Nat Commun. 2023. PMID: 37291098 Free PMC article.
-
Luminescence and Length Control in Nonchelated d8 -Metallosupramolecular Polymers through Metal-Metal Interactions.Angew Chem Int Ed Engl. 2022 Sep 19;61(38):e202208436. doi: 10.1002/anie.202208436. Epub 2022 Aug 17. Angew Chem Int Ed Engl. 2022. PMID: 35749048 Free PMC article.
-
Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications.Molecules. 2024 May 15;29(10):2321. doi: 10.3390/molecules29102321. Molecules. 2024. PMID: 38792182 Free PMC article. Review.
-
Controlling Molecular Packing in Aqueous Metallosupramolecular Self-assembly by Ligand Geometry.Precis Chem. 2023 Jun 15;1(5):332-340. doi: 10.1021/prechem.3c00058. eCollection 2023 Jul 24. Precis Chem. 2023. PMID: 40880891 Free PMC article.
References
-
- Ashton P. R. Brown C. L. Chrystal E. J. T. Goodnow T. T. Kaifer A. E. Parry K. P. Philp D. Slawin A. M. Z. Spencer N. Stoddart J. F. Williams D. J. J. Chem. Soc., Chem. Commun. 1991:634. doi: 10.1039/C39910000634. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

