Two-step anti-cooperative self-assembly process into defined π-stacked dye oligomers: insights into aggregation-induced enhanced emission
- PMID: 34603660
- PMCID: PMC8480337
- DOI: 10.1039/d1sc03813c
Two-step anti-cooperative self-assembly process into defined π-stacked dye oligomers: insights into aggregation-induced enhanced emission
Abstract
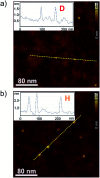
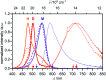
Aggregation-induced emission enhancement (AIEE) phenomena received great popularity during the last decade but in most cases insights into the packing structure - fluorescence properties remained scarce. Here, an almost non-fluorescent merocyanine dye was equipped with large solubilizing substituents, which allowed the investigation of it's aggregation behaviour in unpolar solvents over a large concentration range (10-2 to 10-7 M). In depth analysis of the self-assembly process by concentration-dependent UV/Vis spectroscopy at different temperatures revealed a two-step anti-cooperative aggregation mechanism. In the first step a co-facially stacked dimer is formed driven by dipole-dipole interactions. In a second step these dimers self-assemble to give an oligomer stack consisting of about ten dyes. Concentration- and temperature-dependent UV/Vis spectroscopy provided insight into the thermodynamic parameters and allowed to identify conditions where either the monomer, the dimer or the decamer prevails. The centrosymmetric dimer structure could be proven by 2D NMR spectroscopy. For the larger decamer atomic force microscopy (AFM), diffusion ordered spectroscopy (DOSY) and vapour pressure osmometric (VPO) measurements consistently indicated that it is of small and defined size. Fluorescence, circular dichroism (CD) and circularly polarized luminescence (CPL) spectroscopy provided insights into the photofunctional properties of the dye aggregates. Starting from an essentially non-fluorescent monomer (Φ Fl = 0.23%) a strong AIEE effect with excimer-type fluorescence (large Stokes shift, increased fluorescence lifetime) is observed upon formation of the dimer (Φ Fl = 2.3%) and decamer (Φ Fl = 4.5%) stack. This increase in fluorescence is accompanied for both aggregates by an aggregation-induced CPL enhancement with a strong increase of the g lum from ∼0.001 for the dimer up to ∼0.011 for the higher aggregate. Analysis of the radiative and non-radiative decay rates corroborates the interpretation that the AIEE effect originates from a pronounced decrease of the non-radiative rate due to π-π-stacking induced rigidification that outmatches the effect of the reduced radiative rate that originates from the H-type exciton coupling in the co-facially stacked dyes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Brixner T. Hildner R. Köhler J. Lambert C. Würthner F. Adv. Energy Mater. 2017;7:1700236. doi: 10.1002/aenm.201700236. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

