A novel aggregation-induced enhanced emission aromatic molecule: 2-aminophenylboronic acid dimer
- PMID: 34603674
- PMCID: PMC8480421
- DOI: 10.1039/d1sc03765j
A novel aggregation-induced enhanced emission aromatic molecule: 2-aminophenylboronic acid dimer
Abstract
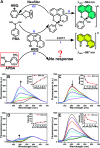
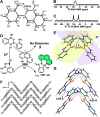
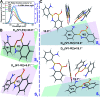
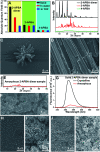
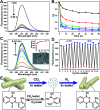
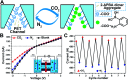
Aggregation-induced enhanced emission (AIEE) molecules have significant applications in optoelectronics, biomedical probes and chemical sensors, and large amounts of AIEE molecules have been reported since the concept of AIEE was proposed. Most aromatic AIEE molecules have complex structures consisting of multiple aromatic rings and/or polycyclic skeletons. In this study, we find that 2-aminophenylboronic acid (2-APBA) with a simple structure is highly emissive in the solid state. Further studies reveal that 2-APBA exists in a dimeric form, and the 2-APBA dimer is a novel AIEE molecule. The underlying AIEE mechanism is that the 2-APBA dimeric units aggregate through intermolecular interactions to produce highly ordered molecular packing without the presence of π-π stacking interactions that would lead to aggregation-caused quenching. Furthermore, the 2-APBA dimer aggregates could reversibly transform into its non-fluorescent monomer form driven by new kinds of dynamic covalent B-N and B-O bonds, illustrating its good potential in molecular recognition, nanogating, chemo/bio-sensing and controlled drug release.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Yang J. Fang M. Li Z. Aggregate. 2020;1:6–18. doi: 10.1002/agt2.2. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

