The prognostic value of thyroid-stimulating immunoglobulin in the management of Graves' disease
- PMID: 34603682
- PMCID: PMC8481717
- DOI: 10.1177/20420188211044943
The prognostic value of thyroid-stimulating immunoglobulin in the management of Graves' disease
Abstract
Background: The bioassay of thyroid-stimulating immunoglobulin was reported to have a similar performance to the commonly used thyroid-stimulating hormone binding inhibition assay, also known as thyroid receptor antibody assay. The normal reference range of thyroid receptor antibody levels indicates the withdrawal of anti-thyroid drugs in the recent clinical guidelines.
Methods: A prospective, longitudinal observational study was conducted to evaluate the prognostic value of thyroid-stimulating immunoglobulin in patients with Graves' disease.
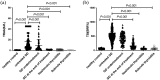
Results: A total of 77 patients with Graves' disease treated with anti-thyroid drugs were in a continuous follow-up until 1 year after anti-thyroid drugs discontinuation. Commercial kits of thyroid-stimulating immunoglobulin and M22-thyroid-stimulating hormone binding inhibition assay were used and compared. Thyroid-stimulating immunoglobulin was all negative in healthy controls, Hashimoto thyroiditis, and subacute thyroiditis. Thyroid-stimulating immunoglobulin value was highest in untreated patients with Graves' disease (p < 0.001). Under anti-thyroid drugs treatment, thyroid-stimulating immunoglobulin value decreased gradually. A total of 21 patients had positive thyroid-stimulating immunoglobulin at the end of treatment. According to clinical fate of patients with Graves' disease after withdrawal of anti-thyroid drugs, thyroid-stimulating immunoglobulin value and positivity in patients with relapse were significantly higher than that reported in patients with remission (p = 0.001, p < 0.001). After adjustment for age, gender, initial thyroid receptor antibody, initial thyroid-stimulating immunoglobulin, and thyroid receptor antibody at the end of treatment, the odds ratio of positive thyroid-stimulating immunoglobulin for the risk of relapse was 33.271 (95% confidence interval: 4.741-233.458, p < 0.001) and odds ratio of quantitative thyroid-stimulating immunoglobulin was 1.009 (95% confidence interval: 1.002-1.015, p < 0.001).
Conclusion: Thyroid-stimulating immunoglobulin is a good predictor of relapse in patients with Graves' disease treated with anti-thyroid drugs. It might be safer to discontinue anti-thyroid drugs when thyroid-stimulating immunoglobulin and thyroid receptor antibody were both negative.
Keywords: Graves’ disease; autoimmune thyroiditis; thyroid-stimulating immunoglobulin; thyrotoxicosis; thyrotropin receptor antibody.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Weetman AP.Graves’ disease. N Engl J Med 2000; 17: 1236–1248. - PubMed
-
- Ando T, Latif R, Davies TF.Thyrotropin receptor antibodies: new insights into their actions and clinical relevance. Best Pract Res Clin Endocrinol Metab 2005; 19: 33–52. - PubMed
-
- Ajjan RA, Weetman AP.Techniques to quantify TSH receptor antibodies. Nat Clin Pract Endocrinol Metab 2008; 4: 461–468. - PubMed
-
- Ross DS, Burch HB, Cooper DS, et al.. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016; 26: 1343–1421. - PubMed
LinkOut - more resources
Full Text Sources

