Porous tantalum-composited gelatin nanoparticles hydrogel integrated with mesenchymal stem cell-derived endothelial cells to construct vascularized tissue in vivo
- PMID: 34603743
- PMCID: PMC8481010
- DOI: 10.1093/rb/rbab051
Porous tantalum-composited gelatin nanoparticles hydrogel integrated with mesenchymal stem cell-derived endothelial cells to construct vascularized tissue in vivo
Abstract
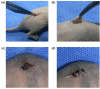
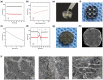
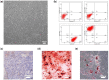
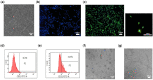
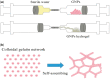
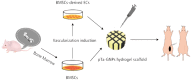
The ideal scaffold material of angiogenesis should have mechanical strength and provide appropriate physiological microporous structures to mimic the extracellular matrix environment. In this study, we constructed an integrated three-dimensional scaffold material using porous tantalum (pTa), gelatin nanoparticles (GNPs) hydrogel, and seeded with bone marrow mesenchymal stem cells (BMSCs)-derived endothelial cells (ECs) for vascular tissue engineering. The characteristics and biocompatibility of pTa and GNPs hydrogel were evaluated by mechanical testing, scanning electron microscopy, cell counting kit, and live-cell assay. The BMSCs-derived ECs were identified by flow cytometry and angiogenesis assay. BMSCs-derived ECs were seeded on the pTa-GNPs hydrogel scaffold and implanted subcutaneously in nude mice. Four weeks after the operation, the scaffold material was evaluated by histomorphology. The superior biocompatible ability of pTa-GNPs hydrogel scaffold was observed. Our in vivo results suggested that 28 days after implantation, the formation of the stable capillary-like network in scaffold material could be promoted significantly. The novel, integrated pTa-GNPs hydrogel scaffold is biocompatible with the host, and exhibits biomechanical and angiogenic properties. Moreover, combined with BMSCs-derived ECs, it could construct vascular engineered tissue in vivo. This study may provide a basis for applying pTa in bone regeneration and autologous BMSCs in tissue-engineered vascular grafts.
Keywords: bone marrow mesenchymal stem cell; endothelial cell; gelatin nanoparticles hydrogel; porous tantalum; vascularization.
© The Author(s) 2021. Published by Oxford University Press.
Figures




Similar articles
-
Mesenchymal stem cell-loaded porous tantalum integrated with biomimetic 3D collagen-based scaffold to repair large osteochondral defects in goats.Stem Cell Res Ther. 2019 Mar 5;10(1):72. doi: 10.1186/s13287-019-1176-2. Stem Cell Res Ther. 2019. PMID: 30837004 Free PMC article.
-
Tantalum coating of porous carbon scaffold supplemented with autologous bone marrow stromal stem cells for bone regeneration in vitro and in vivo.Exp Biol Med (Maywood). 2016 Mar;241(6):592-602. doi: 10.1177/1535370216629578. Epub 2016 Feb 2. Exp Biol Med (Maywood). 2016. PMID: 26843518 Free PMC article.
-
[Influence of the stiffness of three-dimensionally bioprinted extracellular matrix analogue on the differentiation of bone mesenchymal stem cells into skin appendage cells].Zhonghua Shao Shang Za Zhi. 2020 Nov 20;36(11):1013-1023. doi: 10.3760/cma.j.cn501120-20200811-00375. Zhonghua Shao Shang Za Zhi. 2020. PMID: 33238684 Chinese.
-
3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects.Mater Today Bio. 2022 Aug 8;16:100382. doi: 10.1016/j.mtbio.2022.100382. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36033373 Free PMC article.
-
Mesenchymal stem cell-seeded porous tantalum-based biomaterial: A promising choice for promoting bone regeneration.Colloids Surf B Biointerfaces. 2022 Jul;215:112491. doi: 10.1016/j.colsurfb.2022.112491. Epub 2022 Apr 6. Colloids Surf B Biointerfaces. 2022. PMID: 35405535 Review.
Cited by
-
Porous metal materials for applications in orthopedic field: A review on mechanisms in bone healing.J Orthop Translat. 2024 Oct 11;49:135-155. doi: 10.1016/j.jot.2024.08.003. eCollection 2024 Nov. J Orthop Translat. 2024. PMID: 40226784 Free PMC article. Review.
-
Transition metals in angiogenesis - A narrative review.Mater Today Bio. 2023 Aug 3;22:100757. doi: 10.1016/j.mtbio.2023.100757. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37593220 Free PMC article. Review.
-
Recent advances in regenerative biomaterials.Regen Biomater. 2022 Dec 5;9:rbac098. doi: 10.1093/rb/rbac098. eCollection 2022. Regen Biomater. 2022. PMID: 36518879 Free PMC article. Review.
-
Functional hydrogel empowering 3D printing titanium alloys.Mater Today Bio. 2024 Dec 24;30:101422. doi: 10.1016/j.mtbio.2024.101422. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39830135 Free PMC article. Review.
-
Tantalum as Trabecular Metal for Endosseous Implantable Applications.Biomimetics (Basel). 2023 Jan 23;8(1):49. doi: 10.3390/biomimetics8010049. Biomimetics (Basel). 2023. PMID: 36810380 Free PMC article. Review.
References
-
- Jain RK, Au P, Tam J. et al.Engineering vascularized tissue. Nat Biotechnol 2005;23:821–3. - PubMed
-
- Shieh SJ, Vacanti JP.. State-of-the-art tissue engineering: from tissue engineering to organ building. Surgery 2005;137:1–7. - PubMed
-
- Chrobak KM, Potter DR, Tien J.. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 2006;71:185–96. - PubMed
-
- Won J, Yun Y, Jang J. et al.Multifunctional and stable bone mimic proteinaceous matrix for bone tissue engineering. Biomaterials 2015;56:46–57. - PubMed
LinkOut - more resources
Full Text Sources

