Vitamin D/VDR regulates peripheral energy homeostasis via central renin-angiotensin system
- PMID: 34603779
- PMCID: PMC8463910
- DOI: 10.1016/j.jare.2021.01.011
Vitamin D/VDR regulates peripheral energy homeostasis via central renin-angiotensin system
Abstract
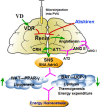
Introduction: Some epidemiological studies have revealed that vitamin D (VD) deficiency is closely linked with the prevalence of obesity, however, the role of VD in energy homeostasis is yet to be investigated, especially in central nervous system. Given that VD negatively regulates renin in adipose tissue, we hypothesized that central VD might play a potential role in energy homeostasis.
Objectives: The present study aims to investigate the potential role of VD in energy homeostasis in the CNS and elaborate its underlying mechanisms.
Methods: This study was conducted in Cyp27b1-/- mice, VD-treated and wild-type mice. After the intraventricular injection of renin or its inhibitors, the changes of renin-angiotensin system (RAS) and its down-stream pathway as well as their effects on metabolic rate were examined.
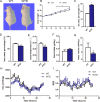
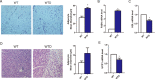
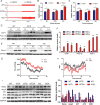
Results: The RAS activity was enhanced in Cyp27b1-/- mice, exhibiting a increased metabolic rate. Additionally, corticotropin-releasing hormone (CRH), a RAS-mediated protein regulating energy metabolism in the hypothalamus, increased significantly in Cyp27b1-/- mice. While in VD-treated group, the RAS and sympathetic nerve activities were slightly inhibited, hence the reduced metabolic rate.
Conclusion: Collectively, the present study demonstrates that the VD/vitamin D receptor (VDR) has a significant impact on energy homeostasis through the modulation of RAS activity in the hypothalamus, subsequently altering CRH expression and sympathetic nervous activity.
Keywords: Central nervous system; Corticotropin-releasing hormone; Energy homeostasis; Renin-angiotensin system; Vitamin D.
© 2021 The Authors. Published by Elsevier B.V. on behalf of Cairo University. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Losartan and Vitamin D Inhibit Colonic Tumor Development in a Conditional Apc-Deleted Mouse Model of Sporadic Colon Cancer.Cancer Prev Res (Phila). 2019 Jul;12(7):433-448. doi: 10.1158/1940-6207.CAPR-18-0380. Epub 2019 May 14. Cancer Prev Res (Phila). 2019. PMID: 31088824 Free PMC article.
-
The renin-angiotensin system mediates EGF receptor-vitamin d receptor cross-talk in colitis-associated colon cancer.Clin Cancer Res. 2014 Nov 15;20(22):5848-5859. doi: 10.1158/1078-0432.CCR-14-0209. Epub 2014 Sep 11. Clin Cancer Res. 2014. PMID: 25212605 Free PMC article.
-
A novel role for vitamin D: modulation of expression and function of the local renin-angiotensin system in mouse pancreatic islets.Diabetologia. 2011 Aug;54(8):2077-81. doi: 10.1007/s00125-011-2100-1. Epub 2011 Mar 20. Diabetologia. 2011. PMID: 21424540
-
Vitamin D/VDR in the pathogenesis of intervertebral disc degeneration: Does autophagy play a role?Biomed Pharmacother. 2022 Apr;148:112739. doi: 10.1016/j.biopha.2022.112739. Epub 2022 Feb 24. Biomed Pharmacother. 2022. PMID: 35202910 Review.
-
Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure.J Steroid Biochem Mol Biol. 2004 May;89-90(1-5):387-92. doi: 10.1016/j.jsbmb.2004.03.004. J Steroid Biochem Mol Biol. 2004. PMID: 15225806 Review.
Cited by
-
The role of interaction between vitamin D and VDR FokI gene polymorphism (rs2228570) in sleep quality of adults.Sci Rep. 2024 Apr 7;14(1):8141. doi: 10.1038/s41598-024-58561-2. Sci Rep. 2024. PMID: 38584183 Free PMC article.
-
A pilot clinical trial to explore the effects of UV exposure on vitamin D synthesis and inflammatory responses in vitamin D-Deficient adults.Sci Rep. 2025 Jul 2;15(1):22557. doi: 10.1038/s41598-025-09203-8. Sci Rep. 2025. PMID: 40595199 Free PMC article. Clinical Trial.
-
Effects of vitamin D supplementation on depression and some selected pro-inflammatory biomarkers: a double-blind randomized clinical trial.BMC Psychiatry. 2022 Nov 11;22(1):694. doi: 10.1186/s12888-022-04305-3. BMC Psychiatry. 2022. PMID: 36368945 Free PMC article. Clinical Trial.
-
Confronting the global obesity epidemic: investigating the role and underlying mechanisms of vitamin D in metabolic syndrome management.Front Nutr. 2024 Aug 9;11:1416344. doi: 10.3389/fnut.2024.1416344. eCollection 2024. Front Nutr. 2024. PMID: 39183985 Free PMC article. Review.
-
Bariatric Surgery and Vitamin D: Trends in Older Women and Association with Clinical Features and VDR Gene Polymorphisms.Nutrients. 2023 Feb 4;15(4):799. doi: 10.3390/nu15040799. Nutrients. 2023. PMID: 36839157 Free PMC article.
References
-
- Momose K., Inui A., Asakawa A., Ueno N., Nakajima M., Fujimiya M., Kasuga M. Intracerebroventricularly administered corticotropin-releasing factor inhibits food intake and produces anxiety-like behaviour at very low doses in mice. Diab Obes Metab. 1999;1:281–284. - PubMed
-
- LeFeuvre R.A., Rothwell N.J., Stock M.J. Activation of brown fat thermogenesis in response to central injection of corticotropin releasing hormone in the rat. Neuropharmacology. 1987;26:1217–1221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

