Longxuetongluo Capsule protects against cerebral ischemia/reperfusion injury through endoplasmic reticulum stress and MAPK-mediated mechanisms
- PMID: 34603791
- PMCID: PMC8463917
- DOI: 10.1016/j.jare.2021.01.016
Longxuetongluo Capsule protects against cerebral ischemia/reperfusion injury through endoplasmic reticulum stress and MAPK-mediated mechanisms
Abstract
Introduction: Longxuetongluo Capsule (LTC) is wildly applied to treat ischemic stroke in clinical practice in China. However, the pharmacological mechanism of LTC on ischemic stroke is still unstated.
Objective: Our research was designed to study the protective effect of LTC against cerebral ischemia-reperfusion (I/R) injury and reveal the underlying mechanism both in vivo and in vitro.
Methods: PC12 cells treated with glucose deprivation/reperfusion (OGD/R) were used to simulate in vitro ischemia/reperfusion (I/R) injury. The cell viability, apoptosis rate, and protein expressions of PC12 cells were evaluated. In vivo validation of the protective effect of LTC was carried out by middle cerebral artery occlusion (MCAO)/reperfusion treatment, and the underlying mechanism of its anti-apoptosis ability was further revealed by immunohistochemistry staining and Western blotting.
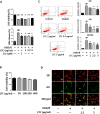
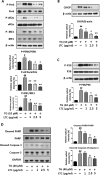
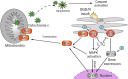
Results: In the current study, we observed that LTC effectively inhibited oxygen-glucose deprivation/reperfusion (OGD/R) induced apoptosis of PC12 cells through suppressing the cleavage of poly ADP-ribose polymerase (PARP), caspase-3, and caspase-9. Further investigation revealed that OGD/R insult remarkably triggered the endoplasmic reticulum stress responses (ER stress) to induce PC12 cell apoptosis. LTC treatment alleviated OGD/R induced ER stress by inhibiting the activation of protein kinase RNA (PKR)-like ER kinase (PERK)/eukaryotic translation initiation factor 2 (eIF2α) and inositol requiring enzyme 1 (IRE1)/tumor necrosis factor receptor-associated factor 2 (TRAF2) pathways. Additionally, LTC also restrained the OGD/R-induced PC12 cell apoptosis by reversing the activated mitogen-activated protein kinase (MAPK) through IRE1/TRAF2 pathway. Animal studies demonstrated LTC significantly restricted the infarct region induced by middle cerebral artery occlusion (MCAO)/reperfusion, the activation of ER stress and apoptosis of neuronal cells had also been suppressed by LTC in the penumbra region.
Conclusion: LTC protects the cerebral neuronal cell against ischemia/reperfusion injury through ER stress and MAPK-mediated mechanisms.
Keywords: Apoptosis; Endoplasmic reticulum stress; Longxuetongluo Capsule; Oxygen-glucose deprivation/reperfusion.
© 2021 The Authors. Published by Elsevier B.V. on behalf of Cairo University. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Marciniec M., Sapko K., Kulczynski M., Popek-Marciniec S., Szczepanska-Szerej A., Rejdak K. Non-traumatic cervical artery dissection and ischemic stroke: a narrative review of recent research. Clin Neurol Neurosur. 2019;187 - PubMed
-
- Perez-de-Puig I., Miro-Mur F., Ferrer-Ferrer M., Gelpi E., Pedragosa J., Justicia C. Neutrophil recruitment to the brain in mouse and human ischemic stroke. ACTA Neuropathol. 2015;129:239–257. - PubMed
-
- Wei K., Wan L., Liu J., Zhang B., Li X., Zhang Y. Downregulation of Trb3 protects neurons against apoptosis induced by global cerebral ischemia and reperfusion Injury in rats. Neuroscience. 2017;360:118–127. - PubMed
-
- Shu Q., Fan H., Li S.J., Zhou D., Ma W., Zhao X.Y. Protective effects of Progranulin against focal cerebral ischemia-reperfusion injury in rats by suppressing endoplasmic reticulum stress and NF-kappa B activation in reactive astrocytes. J Cell Biochem. 2018;119:6584–6597. - PubMed
-
- Pluquet O., Pourtier A., Abbadie C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol-Cell Ph. 2015;308:415–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

