A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus)
- PMID: 34603914
- PMCID: PMC8448810
- DOI: 10.1007/s13205-021-02984-5
A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus)
Abstract
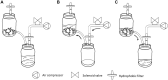
Scaling-up techniques in temporary immersion systems are an alternative for commercial micropropagation. In vitro propagation of pitahaya (Hylocereus undatus) using temporary immersion with liquid culture medium improves micropropagation efficiency compared to the conventional method in semisolid medium. The objective of this work was to evaluate the efficiency of traditional culture and temporary immersion during micropropagation of pitahaya to facilitate the rapid establishment of new commercial plantations of high genetic and phytosanitary quality. Semisolid culture, liquid media in partial immersion and temporary immersion in an Ebb-and-Flow bioreactor were evaluated. Also, in temporary immersion, different immersion frequencies (every 4, 8, 12, and 16 h) and culture densities (5, 10, 15 and 20 explants per bioreactor) were evaluated. For the multiplication stage, new shoots and length were recorded per explant at 45 d of in vitro culture and in the acclimatization stage, the survival percentage was determined at 30 d of greenhouse cultivation. A temporary immersion of 2 min every 4 h and 15 explants per bioreactor was the best culture system, obtaining on average 10.7 shoots per explant with a length of 1.9 cm. No significant differences were observed among treatments during acclimatization, obtaining survival percentages of 98%-100%. This study reports for the first time a protocol for scaling-up techniques in temporary immersion for commercial micropropagation of pitahaya (and for any species of the Cactaceae family) and its establishment in a productive plantation.
Keywords: Bioreactor system; Cactaceae; Dragon fruit; In vitro culture; Semisolid medium.
© King Abdulaziz City for Science and Technology 2021.
Conflict of interest statement
Conflict of interestThe author(s) declare that they have no conflicting interests.
Figures


Similar articles
-
Assessment of different temporary immersion systems in the micropropagation of anthurium (Anthurium andreanum).3 Biotech. 2019 Aug;9(8):307. doi: 10.1007/s13205-019-1833-2. Epub 2019 Jul 26. 3 Biotech. 2019. PMID: 31355116 Free PMC article.
-
Temporary Immersion Systems in Plant Micropropagation.Methods Mol Biol. 2024;2759:3-8. doi: 10.1007/978-1-0716-3654-1_1. Methods Mol Biol. 2024. PMID: 38285134
-
Micropropagation of Guarianthe skinneri (Bateman) Dressler et W. E. Higging in Temporary Immersion Systems.3 Biotech. 2020 Jan;10(1):26. doi: 10.1007/s13205-019-2010-3. Epub 2020 Jan 3. 3 Biotech. 2020. PMID: 31938685 Free PMC article.
-
Orchid Micropropagation Using Temporary Immersion Systems: A Review.Methods Mol Biol. 2024;2759:227-244. doi: 10.1007/978-1-0716-3654-1_21. Methods Mol Biol. 2024. PMID: 38285154 Review.
-
Use of bioreactor systems in the propagation of forest trees.Eng Life Sci. 2019 Sep 30;19(12):896-915. doi: 10.1002/elsc.201900041. eCollection 2019 Dec. Eng Life Sci. 2019. PMID: 32624981 Free PMC article. Review.
Cited by
-
Temporary immersion systems induce photomixotrophism during in vitro propagation of agave Tobalá.3 Biotech. 2024 Mar;14(3):74. doi: 10.1007/s13205-024-03928-5. Epub 2024 Feb 14. 3 Biotech. 2024. PMID: 38371904 Free PMC article.
-
Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit.Plants (Basel). 2023 Feb 24;12(5):1037. doi: 10.3390/plants12051037. Plants (Basel). 2023. PMID: 36903898 Free PMC article.
-
Somatic embryogenesis of Arabica coffee in temporary immersion culture: Advances, limitations, and perspectives for mass propagation of selected genotypes.Front Plant Sci. 2022 Oct 6;13:994578. doi: 10.3389/fpls.2022.994578. eCollection 2022. Front Plant Sci. 2022. PMID: 36275513 Free PMC article.
-
Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions.Plants (Basel). 2023 Nov 22;12(23):3932. doi: 10.3390/plants12233932. Plants (Basel). 2023. PMID: 38068570 Free PMC article.
-
In Vitro Multiplication of Agave (A. marmorata and A. potatorum) by Temporary Immersion in SETIS™ Bioreactor.Methods Mol Biol. 2024;2759:69-76. doi: 10.1007/978-1-0716-3654-1_7. Methods Mol Biol. 2024. PMID: 38285140
References
-
- Aragón CE, Sánchez C, Gonzalez-Olmedo J, Escalona M, Carvalho L, Amâncio S. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol Plant. 2014;58:29–38. doi: 10.1007/s10535-013-0381-6. - DOI
-
- Arano-Avalos S, Gómez-Merino FC, Mancilla-Álvarez E, Sánchez-Páez R, Bello-Bello JJ. An efficient protocol for commercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci Hortic. 2020;261:108998. doi: 10.1016/j.scienta.2019.108998. - DOI
-
- Arigundam U, Variyath AM, Siow YL, Marshall D, Debnath SC. Liquid culture for efficient in vitro propagation of adventitious shoots in wild Vaccinium vitis-idaea ssp. minus (lingonberry) using temporary immersion and stationary bioreactors. Sci Hortic. 2020;264:109199. doi: 10.1016/j.scienta.2020.109199. - DOI
-
- Bello-Bello JJ, Cruz-Cruz CA, Pérez-Guerra JC. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine) In Vitro Cell Dev Biol Plant. 2019;55:313–320. doi: 10.1007/s11627-019-09973-7. - DOI
LinkOut - more resources
Full Text Sources

