Optimization of a food industry-waste-based medium for the production of the plant growth promoting microorganism Pseudomonas oryzihabitans PGP01 based on agro-food industries by-products
- PMID: 34603978
- PMCID: PMC8473457
- DOI: 10.1016/j.btre.2021.e00675
Optimization of a food industry-waste-based medium for the production of the plant growth promoting microorganism Pseudomonas oryzihabitans PGP01 based on agro-food industries by-products
Abstract
In this study, three wastes based on potato peels and pulps, tomato seeds and wheat bran were used as basis for the preparation of a cheap medium to produce the bacterium P. oryzihabitans PGP01. In flasks experiments, P. oryzihabitans PGP01 growth at 25 °C in a medium based on frozen potato peels and pulp (FPP) with tryptone as a nitrogen source resulted in the maximum production compared to the commercial TSB medium. In the scale-up to 2 L bioreactors, FPP supplemented with tryptone, molasses, NaCl and K2HPO4 allowed to reach similar biomass production than in the TSB medium. A maximum growth of 4.4 × 109 CFU mL-1 after setting the agitation and the air flux conditions at 400 rpm and 0.75 vvm. Finally, P. oryzihabitans PGP01 growing in this optimized medium conserved its biological activity showing the expected effect in root development previously reported for this microorganism.
Keywords: Agro-food by-products; In vitro micropropagated plants; Low-cost production; Molasses; Plant growth-promoting microorganisms.
© 2021 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
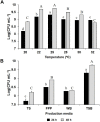
 ) and high (
) and high ( ) concentration. P. oryzihabitans PGP01 cells were cultured at 25°C and 150 rpm. PEP = Peptone, PS = PROSTAR 510A, YE = Yeast extract, ME = Meat extract, PP = Pea protein, MP = Maize protein, TRP = Tryptone. Initial pH of all media were adjusted to 7 by the addition of K2HPO4 0.2 M. Data represent mean ± SE of three independent replicates, and different letters indicate significant differences between TSB and different media according to Tukey HSD test (P < 0.05).
) concentration. P. oryzihabitans PGP01 cells were cultured at 25°C and 150 rpm. PEP = Peptone, PS = PROSTAR 510A, YE = Yeast extract, ME = Meat extract, PP = Pea protein, MP = Maize protein, TRP = Tryptone. Initial pH of all media were adjusted to 7 by the addition of K2HPO4 0.2 M. Data represent mean ± SE of three independent replicates, and different letters indicate significant differences between TSB and different media according to Tukey HSD test (P < 0.05).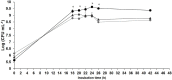
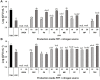
 ) and in media based on potato wastes (FPP) supplemented with 10 g L−1 (
) and in media based on potato wastes (FPP) supplemented with 10 g L−1 ( ) or 20 g L−1 of TRP (
) or 20 g L−1 of TRP ( ) in a 2 L bioreactor at 25°C and 400 rpm. Data represent means ± SE of three independent replicates, and asterisks symbols (*) denote significant differences between commercial and by-products optimized media according to Tukey HSD test (P < 0.05).
) in a 2 L bioreactor at 25°C and 400 rpm. Data represent means ± SE of three independent replicates, and asterisks symbols (*) denote significant differences between commercial and by-products optimized media according to Tukey HSD test (P < 0.05).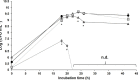
 ) and in media based on frozen potato peels and pulps and 10 g L−1 of TRP (FPP + TRP10) supplemented with 5 g L−1 of NaCl (
) and in media based on frozen potato peels and pulps and 10 g L−1 of TRP (FPP + TRP10) supplemented with 5 g L−1 of NaCl ( ), 5 g L−1 of NaCl, 5 g L−1 of GLU (
), 5 g L−1 of NaCl, 5 g L−1 of GLU ( ) and 5 g L−1 of NaCl, 10 g L−1 of MOL (
) and 5 g L−1 of NaCl, 10 g L−1 of MOL ( ) at 25°C and 400 rpm. TSB Data represents the mean ± SE of three independent replicates. Asterisks (*) symbols within each sampling period denote significant differences between TSB and optimized media according to Tukey HSD test (P < 0.05).
) at 25°C and 400 rpm. TSB Data represents the mean ± SE of three independent replicates. Asterisks (*) symbols within each sampling period denote significant differences between TSB and optimized media according to Tukey HSD test (P < 0.05).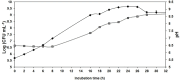
 ) and pH (
) and pH ( ) evolution of growth curve in a 2-L bioreactor of the bacterium P. oryzihabitans PGP01 in the optimized-wastes-medium based on frozen peels and pulps (FPP*: 300 g L−1 FPP, 10 g L− 1 TRP, 10 g L−1 MOL and 5 g L−1 NaCl) at 25°C, 400 rpm and 0.75 vvm of oxygen flux. Initial pH of the culture medium was adjusted to 7 by the addition of K2HPO4 0.2 M. Each value of the log (CFU mL−1) of the curve represents the mean ± SE of at least three replicates.
) evolution of growth curve in a 2-L bioreactor of the bacterium P. oryzihabitans PGP01 in the optimized-wastes-medium based on frozen peels and pulps (FPP*: 300 g L−1 FPP, 10 g L− 1 TRP, 10 g L−1 MOL and 5 g L−1 NaCl) at 25°C, 400 rpm and 0.75 vvm of oxygen flux. Initial pH of the culture medium was adjusted to 7 by the addition of K2HPO4 0.2 M. Each value of the log (CFU mL−1) of the curve represents the mean ± SE of at least three replicates.
Similar articles
-
Rhizosphere microorganisms enhance in vitro root and plantlet development of Pyrus and Prunus rootstocks.Planta. 2021 Mar 14;253(4):78. doi: 10.1007/s00425-021-03595-3. Planta. 2021. PMID: 33715081
-
Agro-Industrial Wastes for Production of Biosurfactant by Bacillus subtilis ANR 88 and Its Application in Synthesis of Silver and Gold Nanoparticles.Front Microbiol. 2017 Mar 24;8:492. doi: 10.3389/fmicb.2017.00492. eCollection 2017. Front Microbiol. 2017. PMID: 28392783 Free PMC article.
-
Production of arachidonic acid by Mortierella alpina using wastes from potato chips industry.J Appl Microbiol. 2021 May;130(5):1592-1601. doi: 10.1111/jam.14864. Epub 2020 Oct 20. J Appl Microbiol. 2021. PMID: 32975836
-
Efficient biodegradation of organic matter using a thermophilic bacterium and development of a cost-effective culture medium for industrial use.J Environ Sci Health A Tox Hazard Subst Environ Eng. 2020;55(6):686-696. doi: 10.1080/10934529.2020.1732173. Epub 2020 Feb 28. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2020. PMID: 32107954
-
Pullulan production from agro-industrial waste and its applications in food industry: A review.Carbohydr Polym. 2019 Aug 1;217:46-57. doi: 10.1016/j.carbpol.2019.04.050. Epub 2019 Apr 13. Carbohydr Polym. 2019. PMID: 31079684 Review.
Cited by
-
The phyllosphere of Nigerian medicinal plants, Euphorbia lateriflora and Ficus thonningii is inhabited by a specific microbiota.Sci Rep. 2024 Oct 1;14(1):22806. doi: 10.1038/s41598-024-68001-w. Sci Rep. 2024. PMID: 39354019 Free PMC article.
-
Culturomics- and metagenomics-based insights into the soil microbiome preservation and application for sustainable agriculture.Front Microbiol. 2024 Oct 24;15:1473666. doi: 10.3389/fmicb.2024.1473666. eCollection 2024. Front Microbiol. 2024. PMID: 39526137 Free PMC article. Review.
-
The Roles of Plant-Growth-Promoting Rhizobacteria (PGPR)-Based Biostimulants for Agricultural Production Systems.Plants (Basel). 2024 Feb 23;13(5):613. doi: 10.3390/plants13050613. Plants (Basel). 2024. PMID: 38475460 Free PMC article. Review.
References
-
- Collavino M.M., Sansberro P.A., Mroginski L.A., Aguilar O.M. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils. 2010;46:727–738. doi: 10.1007/s00374-010-0480-x. - DOI
-
- Kuzmicheva Y.V., Shaposhnikov A.I., Petrova S.N., Makarova N.M., Tychinskaya I.L., Puhalsky J.V., Parahin N.V., Tikhonovich I.A., Belimov A.A. Variety specific relationships between effects of rhizobacteria on root exudation, growth and nutrient uptake of soybean. Plant Soil. 2017;419:83–96. doi: 10.1007/s11104-017-3320-z. - DOI
-
- Contesto C., Milesi S., Mantelin S., Zancarini A., Desbrosses G., Varoquaux F., Bellini C., Kowalczyk M., Touraine B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta. 2010;232:1455–1470. doi: 10.1007/s00425-010-1264-0. - DOI - PubMed
-
- Iqbal A., Hasnain S. Aeromonas punctata PNS-1: a promising candidate to change the root morphogenesis of Arabidopsis thaliana in MS and sand system. Acta Physiol. Plant. 2013;35:657–665. doi: 10.1007/s11738-012-1106-8. - DOI
LinkOut - more resources
Full Text Sources
