Ultra-high resolution computed tomography of joints: practical recommendations for acquisition protocol optimization
- PMID: 34603984
- PMCID: PMC8408802
- DOI: 10.21037/qims-21-217
Ultra-high resolution computed tomography of joints: practical recommendations for acquisition protocol optimization
Abstract
Background: To assess the influence on the spatial resolution of various Ultra-high-resolution computed tomography (CT) parameters and provide practical recommendations for acquisition protocol optimization in musculoskeletal imaging.
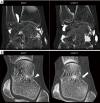
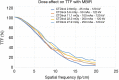
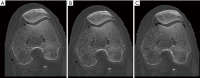
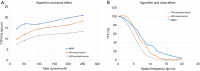
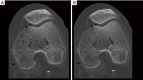
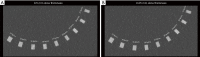
Methods: All acquisitions were performed with an Ultra-high resolution scanner, and variations of the following parameters were evaluated: field-of-view (150-300 mm), potential (80-140 KVp), current (25-250 mAs), focal spot size (0.4×0.5 to 0.8×1.3 mm2), slice thickness (0.25-0.5 mm), reconstruction matrix (512×512 to 2048×2048), and iso-centering (up to 85 mm off-center). Two different image reconstruction algorithms were evaluated: hybrid iterative reconstruction (HIR) and model-based iterative reconstruction (MBIR). CATPHAN 600 phantom images were analyzed to calculate the number of visible line pairs per centimeter (lp/cm). Task transfer function (TTF) curves were calculated to quantitatively evaluate spatial resolution. Cadaveric knee acquisitions were also performed.
Results: Under the conditions studied, the factor that most intensely influenced spatial resolution was the matrix size (additional visualization of up to 8 lp/cm). Increasing the matrix from 512×512 to 2048×2048 led to a 28.2% increase in TTF10% values with a high-dose protocol and a 5.6% increase with a low-dose protocol with no change in the number of visually distinguishable line pairs. The second most important factor affecting spatial resolution was the tube output (29.6% TTF10% gain and 5 additional lp/cm visualized), followed by the reconstruction algorithm choice and lateral displacement (both with a 4 lp/cm gain). Decreasing the slice thickness from 0.5 to 0.25 mm, led to an increase of 3 lp/cm (from 17 to 20 lp/cm) and a 17.3% increase in TTF10% values with no change in the "in-plane" spatial resolution.
Conclusions: This study provides practical recommendations for spatial resolution optimization using Ultra-high-resolution CT.
Keywords: Computed tomography (CT); musculoskeletal imaging; radiation dose; recommendations; spatial resolution.
2021 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-21-217). Two authors involved in this work (P.A.G.T and A.B.) participate in a non-remunerated research contract with Canon Medical Systems, manufacturer of the CT scanner used in this study. The other authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Characteristics and clinical application of a treatment simulator with Ct-option.Radiother Oncol. 1999 Mar;50(3):355-66. doi: 10.1016/s0167-8140(99)00033-x. Radiother Oncol. 1999. PMID: 10392823
-
An evaluation of spatial resolution of a prototype proton CT scanner.Med Phys. 2016 Dec;43(12):6291. doi: 10.1118/1.4966028. Med Phys. 2016. PMID: 27908179 Free PMC article.
-
Quantitative measurements of emphysema in ultra-high resolution computed tomography using model-based iterative reconstruction in comparison to that using hybrid iterative reconstruction.Phys Eng Sci Med. 2022 Mar;45(1):115-124. doi: 10.1007/s13246-021-01091-2. Epub 2022 Jan 13. Phys Eng Sci Med. 2022. PMID: 35023075
-
Full model-based iterative reconstruction (MBIR) in abdominal CT increases objective image quality, but decreases subjective acceptance.Eur Radiol. 2019 Aug;29(8):4016-4025. doi: 10.1007/s00330-018-5988-8. Epub 2019 Jan 30. Eur Radiol. 2019. PMID: 30701327
-
Clinical Application of Ultra-High-Frequency Ultrasound.J Pers Med. 2022 Oct 19;12(10):1733. doi: 10.3390/jpm12101733. J Pers Med. 2022. PMID: 36294872 Free PMC article. Review.
Cited by
-
Photon-counting CT of elbow joint fractures: image quality in a simulated post-trauma setting with off-center positioning.Eur Radiol Exp. 2023 Mar 27;7(1):15. doi: 10.1186/s41747-023-00329-w. Eur Radiol Exp. 2023. PMID: 36967394 Free PMC article.
References
-
- Stok KS, Burghardt AJ, Boutroy S, Peters MPH, Manske SL, Stadelmann V, Vilayphiou N, Van den Bergh JP, Geusens P, Li X, Marotte H, Van Rietbergen B, Boyd S, Barnabe C, SPECTRA Collaboration. Consensus approach for 3D joint space width of metacarpophalangeal joints of rheumatoid arthritis patients using high-resolution peripheral quantitative computed tomography. Quant Imaging Med Surg 2020;10:314-25. 10.21037/qims.2019.12.11 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
