Psychosocial Stress and Age Influence Depression and Anxiety-Related Behavior, Drive Tumor Inflammatory Cytokines and Accelerate Prostate Cancer Growth in Mice
- PMID: 34604038
- PMCID: PMC8481826
- DOI: 10.3389/fonc.2021.703848
Psychosocial Stress and Age Influence Depression and Anxiety-Related Behavior, Drive Tumor Inflammatory Cytokines and Accelerate Prostate Cancer Growth in Mice
Abstract
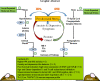
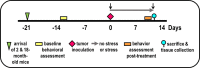
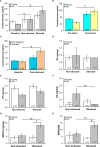
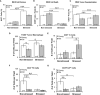
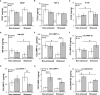
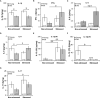
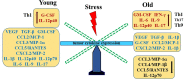
Prostate cancer (PCa) prevalence is higher in older men and poorer coping with psychosocial stressors effect prognosis. Yet, interactions between age, stress and PCa progression are underexplored. Therefore, we characterized the effects of age and isolation combined with restraint (2 h/day) for 14 days post-tumor inoculation on behavior, tumor growth and host defense in the immunocompetent, orthotopic RM-9 murine PCa model. All mice were tumor inoculated. Isolation/restraint increased sympathetic and hypothalamic-pituitary-adrenal cortical activation, based on elevated serum 3-methoxy-4-hydroxyphenylglycol/norepinephrine ratios and corticosterone levels, respectively. Elevated zero maze testing revealed age-related differences in naïve C57Bl/6 mice, and increased anxiety-like behavior in tumor-bearing mice. In open field testing, old stressed mice were less active throughout the 30-min test than young non-stressed and stressed, and old non-stressed mice, suggesting greater anxiety in old stressed mice. Old (18 month) mice demonstrated more depression-like behavior than young mice with tail suspension testing, without effects of isolation/restraint stress. Old mice developed larger tumors, despite similar tumor expression of tumor vascular endothelial growth factor or transforming growth factor-beta1 across age. Tumor chemokine/cytokine expression, commonly prognostic for poorer outcomes, were uniquely age- and stress-dependent, underscoring the need for PCa research in old animals. Macrophages predominated in RM-9 tumors. Macrophages, and CD4+ and CD4+FoxP3+ T-cell tumor infiltration were greater in young mice than in old mice. Stress increased macrophage infiltration in old mice. Conversely, stress reduced intratumoral CD4+ and CD4+FoxP3+ T-cell numbers in young mice. CD8+ T-cell infiltration was similar across treatment groups. Our findings support that age- and psychological stress interacts to affect PCa outcomes by interfering with neural-immune mechanisms and affecting behavioral responses.
Keywords: IL-9/IL-17 balance; aging; anxiety/depression-related behavior; psychosocial stress; tumor immunity.
Copyright © 2021 Bellinger, Dulcich, Molinaro, Gifford, Lorton, Gridley and Hartman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Differential effects of stress exposure via two types of restraint apparatuses on behavior and plasma corticosterone level in inbred male BALB/cAJcl mice.Neuropsychopharmacol Rep. 2020 Mar;40(1):73-84. doi: 10.1002/npr2.12093. Epub 2019 Dec 23. Neuropsychopharmacol Rep. 2020. PMID: 31872573 Free PMC article.
-
The effects of a standardized Acanthopanax koreanum extract on stress-induced behavioral alterations in mice.J Ethnopharmacol. 2013 Jul 30;148(3):826-34. doi: 10.1016/j.jep.2013.05.019. Epub 2013 May 27. J Ethnopharmacol. 2013. PMID: 23721913
-
Tumour necrosis factor receptor deficiency alters anxiety-like behavioural and neuroendocrine stress responses of mice.Cytokine. 2012 Jul;59(1):72-8. doi: 10.1016/j.cyto.2012.04.001. Epub 2012 May 4. Cytokine. 2012. PMID: 22561136
-
Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats.Behav Brain Res. 2010 Aug 25;211(2):169-77. doi: 10.1016/j.bbr.2010.03.025. Epub 2010 Mar 20. Behav Brain Res. 2010. PMID: 20307586
-
CD4+ T helper 17 cell response of aged mice promotes prostate cancer cell migration and invasion.Prostate. 2020 Jul;80(10):764-776. doi: 10.1002/pros.23990. Epub 2020 May 1. Prostate. 2020. PMID: 32356608 Free PMC article.
Cited by
-
β-Glucan ameliorates anxiety-like behavior in mice chronically infected with the Toxoplasma gondii Wh6 strain.Parasitol Res. 2022 Dec;121(12):3513-3521. doi: 10.1007/s00436-022-07675-5. Epub 2022 Sep 27. Parasitol Res. 2022. PMID: 36163518
-
Effects of chronic restraint stress in the prostate of prepubertal and adult rats.Acta Cir Bras. 2023 Dec 4;38:e387123. doi: 10.1590/acb387123. eCollection 2023. Acta Cir Bras. 2023. PMID: 38055386 Free PMC article.
-
The Roles of Tumor-Associated Macrophages in Prostate Cancer.J Oncol. 2022 Sep 7;2022:8580043. doi: 10.1155/2022/8580043. eCollection 2022. J Oncol. 2022. PMID: 36117852 Free PMC article. Review.
-
The role of proinflammatory cytokines and CXC chemokines (CXCL1-CXCL16) in the progression of prostate cancer: insights on their therapeutic management.Cell Mol Biol Lett. 2024 May 14;29(1):73. doi: 10.1186/s11658-024-00591-9. Cell Mol Biol Lett. 2024. PMID: 38745115 Free PMC article. Review.
-
Psychological distress and eustress in cancer and cancer treatment: Advances and perspectives.Sci Adv. 2022 Nov 25;8(47):eabq7982. doi: 10.1126/sciadv.abq7982. Epub 2022 Nov 23. Sci Adv. 2022. PMID: 36417542 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

