Bleeding Risk Following Stereotactic Body Radiation Therapy for Localized Prostate Cancer in Men on Baseline Anticoagulant or Antiplatelet Therapy
- PMID: 34604059
- PMCID: PMC8485025
- DOI: 10.3389/fonc.2021.722852
Bleeding Risk Following Stereotactic Body Radiation Therapy for Localized Prostate Cancer in Men on Baseline Anticoagulant or Antiplatelet Therapy
Abstract
Purpose: Patients on anticoagulant/antiplatelet medications are at a high risk of bleeding following external beam radiation therapy for localized prostate cancer. SBRT may reduce the bleeding risk by decreasing the volume of bladder/rectum receiving high doses. This retrospective study sought to evaluate the rates of hematuria and hematochezia following SBRT in these patients.
Methods: Localized prostate cancer patients treated with SBRT from 2007 to 2017 on at least one anticoagulant/antiplatelet at baseline were included. The minimum follow-up was 3 years with a median follow-up of 72 months. Patients who had a rectal spacer placed prior to SBRT were excluded. Radiotherapy was delivered in 5 fractions to a dose of 35 Gy or 36.25 Gy utilizing the CyberKnife system. Hematuria and hematochezia were prospectively assessed before and after treatment using the Expanded Prostate Cancer Index Composite (EPIC-26). Toxicities were scored using the CTCAE v4. Cystoscopy and colonoscopy findings were retrospectively reviewed.
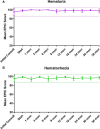
Results: Forty-four men with a median age of 72 years with a history of taking at least one anticoagulant and/or antiplatelet medication received SBRT. Warfarin (46%), clopidogrel (34%) and rivaroxaban (9%) were the most common medications. Overall, 18.2% experienced hematuria with a median time of 10.5 months post-SBRT. Altogether, 38.6% experienced hematochezia with a median time of 6 months post-SBRT. ≥ Grade 2 hematuria and hematochezia occurred in 4.6% and 2.5%, respectively. One patient required bladder neck fulguration and one patient underwent rectal cauterization for multiple non-confluent telangiectasia. There were no grade 4 or 5 toxicities. Cystoscopy revealed bladder cancer (40%) and benign prostatic bleeding (40%) as the most common hematuria etiology. Colonoscopy demonstrated hemorrhoids (54.5%) and radiation proctitis (9.1%) as the main causes of hematochezia. There was no significant change from the mean baseline EPIC-26 hematuria and hematochezia scores at any point during follow up.
Conclusion: In patients with baseline anticoagulant usage, moderate dose prostate SBRT was well tolerated without rectal spacing. High grade bleeding toxicities were uncommon and resolved with time. Baseline anticoagulation usage should not be considered a contraindication to prostate SBRT.
Keywords: anticoagulation; antiplatelet; bleeding risk; prostate cancer; stereotactic body radiation therapy.
Copyright © 2021 Pepin, Shah, Pernia, Lei, Ayoob, Danner, Yung, Collins, Suy, Aghdam and Collins.
Conflict of interest statement
SC and BC serve as clinical consultants to Accuray Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Rationale for Utilization of Hydrogel Rectal Spacers in Dose Escalated SBRT for the Treatment of Unfavorable Risk Prostate Cancer.Front Oncol. 2022 Mar 31;12:860848. doi: 10.3389/fonc.2022.860848. eCollection 2022. Front Oncol. 2022. PMID: 35433457 Free PMC article. Review.
-
Urinary Morbidity in Men Treated With Stereotactic Body Radiation Therapy (SBRT) for Localized Prostate Cancer Following Transurethral Resection of the Prostate (TURP).Front Oncol. 2020 May 5;10:555. doi: 10.3389/fonc.2020.00555. eCollection 2020. Front Oncol. 2020. PMID: 32432033 Free PMC article.
-
Hematuria following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer.Radiat Oncol. 2015 Feb 19;10:44. doi: 10.1186/s13014-015-0351-6. Radiat Oncol. 2015. PMID: 25890265 Free PMC article.
-
Proctitis following stereotactic body radiation therapy for prostate cancer.Radiat Oncol. 2014 Dec 12;9:277. doi: 10.1186/s13014-014-0277-4. Radiat Oncol. 2014. PMID: 25497602 Free PMC article.
-
Stereotactic Body Radiation Therapy (SBRT) for Prostate Cancer in Men With a High Baseline International Prostate Symptom Score (IPSS ≥ 15).Front Oncol. 2020 Jul 3;10:1060. doi: 10.3389/fonc.2020.01060. eCollection 2020. Front Oncol. 2020. PMID: 32719744 Free PMC article.
Cited by
-
Rationale for Utilization of Hydrogel Rectal Spacers in Dose Escalated SBRT for the Treatment of Unfavorable Risk Prostate Cancer.Front Oncol. 2022 Mar 31;12:860848. doi: 10.3389/fonc.2022.860848. eCollection 2022. Front Oncol. 2022. PMID: 35433457 Free PMC article. Review.
References
-
- D’Amico AV, Manola J, McMahon E, Loffredo M, Lopes L, Ching J, et al. . A Prospective Evaluation of Rectal Bleeding After Dose-Escalated Three-Dimensional Conformal Radiation Therapy Using an Intrarectal Balloon for Prostate Gland Localization and Immobilization. Urol (2006) 67(4):780–4. doi: 10.1016/j.urology.2005.10.008 - DOI - PubMed
LinkOut - more resources
Full Text Sources

