Immunotherapeutic Potential of T Memory Stem Cells
- PMID: 34604060
- PMCID: PMC8485052
- DOI: 10.3389/fonc.2021.723888
Immunotherapeutic Potential of T Memory Stem Cells
Abstract
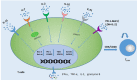
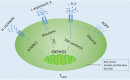
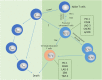
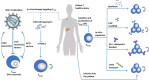
Memory T cells include T memory stem cells (TSCM) and central memory T cells (TCM). Compared with effector memory T cells (TEM) and effector T cells (TEFF), they have better durability and anti-tumor immunity. Recent studies have shown that although TSCM has excellent self-renewal ability and versatility, if it is often exposed to antigens and inflammatory signals, TSCM will behave as a variety of inhibitory receptors such as PD-1, TIM-3 and LAG-3 expression, and metabolic changes from oxidative phosphorylation to glycolysis. These changes can lead to the exhaustion of T cells. Cumulative evidence in animal experiments shows that it is the least differentiated cell in the memory T lymphocyte system and is a central participant in many physiological and pathological processes in humans. It has a good clinical application prospect, so it is more and more important to study the factors affecting the formation of TSCM. This article summarizes and prospects the phenotypic and functional characteristics of TSCM, the regulation mechanism of formation, and its application in treatment of clinical diseases.
Keywords: HIV; T memory stem cells; autoimmune diseases; stemness; tumor immunotherapy.
Copyright © 2021 Li, Wu, Yang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Memory T cell, exhaustion, and tumor immunity.Immunol Med. 2020 Mar;43(1):1-9. doi: 10.1080/25785826.2019.1698261. Epub 2019 Dec 10. Immunol Med. 2020. PMID: 31822213 Review.
-
T memory stem cell characteristics in autoimmune diseases and their promising therapeutic values.Front Immunol. 2023 Jul 11;14:1204231. doi: 10.3389/fimmu.2023.1204231. eCollection 2023. Front Immunol. 2023. PMID: 37497231 Free PMC article. Review.
-
Role of stem cell-like memory T cells in autoimmune diseases.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Jul 28;48(7):1098-1104. doi: 10.11817/j.issn.1672-7347.2023.230051. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37724413 Free PMC article. Chinese, English.
-
T memory stem cells in health and disease.Nat Med. 2017 Jan 6;23(1):18-27. doi: 10.1038/nm.4241. Nat Med. 2017. PMID: 28060797 Free PMC article. Review.
-
Memory CD4+ T cell subsets in tumor draining lymph nodes of breast cancer patients: A focus on T stem cell memory cells.Cell Oncol (Dordr). 2018 Feb;41(1):1-11. doi: 10.1007/s13402-017-0352-6. Epub 2017 Oct 9. Cell Oncol (Dordr). 2018. PMID: 28994018
Cited by
-
Metabolic reprogramming in the tumor microenvironment: unleashing T cell stemness for enhanced cancer immunotherapy.Front Pharmacol. 2023 Dec 19;14:1327717. doi: 10.3389/fphar.2023.1327717. eCollection 2023. Front Pharmacol. 2023. PMID: 38169800 Free PMC article. Review.
-
Cell Immunotherapy against Melanoma: Clinical Trials Review.Int J Mol Sci. 2023 Jan 26;24(3):2413. doi: 10.3390/ijms24032413. Int J Mol Sci. 2023. PMID: 36768737 Free PMC article. Review.
-
Genetically-modified, redirected T cells target hepatitis B surface antigen-positive hepatocytes and hepatocellular carcinoma lesions in a clinical setting.Clin Mol Hepatol. 2024 Oct;30(4):735-755. doi: 10.3350/cmh.2024.0058. Epub 2024 May 29. Clin Mol Hepatol. 2024. PMID: 38808361 Free PMC article.
-
The Potential Use of Digital Twin Technology for Advancing CAR-T Cell Therapy.Curr Issues Mol Biol. 2025 Apr 30;47(5):321. doi: 10.3390/cimb47050321. Curr Issues Mol Biol. 2025. PMID: 40699720 Free PMC article. Review.
-
A strategy to reconstitute immunity without GVHD via adoptive allogeneic Tscm therapy.Front Immunol. 2024 Jul 5;15:1367609. doi: 10.3389/fimmu.2024.1367609. eCollection 2024. Front Immunol. 2024. PMID: 39035005 Free PMC article.
References
-
- Khouri R, Silva-Santos G, Dierckx T, Menezes SM, Decanine D, Theys K, et al. . A Genetic IFN/STAT1/FAS Axis Determines CD4 T Stem Cell Memory Levels and Apoptosis in Healthy Controls and Adult T-Cell Leukemia Patients. Oncoimmunology (2018) 7(5):e1426423. doi: 10.1080/2162402X.2018.1426423 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

