Calmodulin 2 Facilitates Angiogenesis and Metastasis of Gastric Cancer via STAT3/HIF-1A/VEGF-A Mediated Macrophage Polarization
- PMID: 34604066
- PMCID: PMC8479158
- DOI: 10.3389/fonc.2021.727306
Calmodulin 2 Facilitates Angiogenesis and Metastasis of Gastric Cancer via STAT3/HIF-1A/VEGF-A Mediated Macrophage Polarization
Abstract
Background: Tumor-associated macrophages (TAMs) are indispensable to mediating the connections between cells in the tumor microenvironment. In this study, we intended to research the function and mechanism of Calmodulin2 (CALM2) in gastric cancer (GC)-TAM microenvironment.
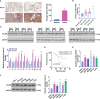
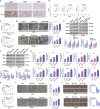
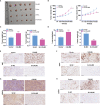
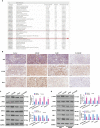
Materials and methods: CALM2 expression in GC tissues and GC cells was determined through quantitative real-time PCR (qRT-PCR) and immunohistochemistry (IHC). The correlation between CALM2 level and the survival rate of GC patients was assessed. The CALM2 overexpression or knockdown model was constructed to evaluate its role in GC cell proliferation, migration, and invasion. THP1 cells or HUVECs were co-cultured with the conditioned medium of GC cells. Tubule formation experiment was done to examine the angiogenesis of endothelial cells. The proliferation, migration, and polarization of THP1 cells were measured. A xenograft model was set up in BALB/c male nude mice to study CALM2x's effects on tumor growth and lung metastasis in vivo. Western Blot (WB) checked the profile of JAK2/STAT3/HIF-1/VEGFA in GC tissues and cells.
Results: In GC tissues and cell lines, CALM2 expression was elevated and positively relevant to the poor prognosis of GC patients. In in-vitro experiments, CALM2 overexpression or knockdown could facilitate or curb the proliferation, migration, invasion, and angiogenesis of HUVECs and M2 polarization of THP1 cells. In in-vivo experiments, CALM2 boosted tumor growth and lung metastasis. Mechanically, CALM2 could arouse the JAK2/STAT3/HIF-1/VEGFA signaling. It was also discovered that JAK2 and HIF-1A inhibition could attenuate the promoting effects of CALM2 on GC, HUVECs cells, and macrophages.
Conclusion: CALM2 modulates the JAK2/STAT3/HIF-1/VEGFA axis and bolsters macrophage polarization, thus facilitating GC metastasis and angiogenesis.
Keywords: angiogenesis; calmodulin 2; cancer; gastric cancer; tumor-associated macrophages.
Copyright © 2021 Mu, Zhu, Dong, Shi, Deng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
[Gastric cancer-derived mesenchymal stem cells regulate the M2 polarization of macrophages within gastric cancer microenvironment via JAK2/STAT3 signaling pathway].Zhonghua Zhong Liu Za Zhi. 2022 Jul 23;44(7):728-736. doi: 10.3760/cma.j.cn112152-20200106-00008. Zhonghua Zhong Liu Za Zhi. 2022. PMID: 35880339 Chinese.
-
HMGA1B/2 transcriptionally activated-POU1F1 facilitates gastric carcinoma metastasis via CXCL12/CXCR4 axis-mediated macrophage polarization.Cell Death Dis. 2021 Apr 29;12(5):422. doi: 10.1038/s41419-021-03703-x. Cell Death Dis. 2021. PMID: 33927188 Free PMC article.
-
Dextran Sulfate Inhibits Angiogenesis and Invasion of Gastric Cancer by Interfering with M2-type Macrophages Polarization.Curr Cancer Drug Targets. 2022;22(11):904-918. doi: 10.2174/1568009622666220705095403. Curr Cancer Drug Targets. 2022. PMID: 35792124
-
Dihydroartemisinin Prevents Progression and Metastasis of Head and Neck Squamous Cell Carcinoma by Inhibiting Polarization of Macrophages in Tumor Microenvironment.Onco Targets Ther. 2020 Apr 22;13:3375-3387. doi: 10.2147/OTT.S249046. eCollection 2020. Onco Targets Ther. 2020. PMID: 32425545 Free PMC article.
-
Helicobacter pylori-induced fibroblast-derived Serpin E1 promotes gastric cancer growth and peritoneal dissemination through p38 MAPK/VEGFA-mediated angiogenesis.Cancer Cell Int. 2023 Dec 16;23(1):326. doi: 10.1186/s12935-023-03177-1. Cancer Cell Int. 2023. PMID: 38104099 Free PMC article.
Cited by
-
Exploring the impact of circRNAs on cancer glycolysis: Insights into tumor progression and therapeutic strategies.Noncoding RNA Res. 2024 May 5;9(3):970-994. doi: 10.1016/j.ncrna.2024.05.001. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38770106 Free PMC article. Review.
-
Transcription factor TCF3 promotes bladder cancer development via TMBIM6-Ca2+-dependent ferroptosis.Cell Death Discov. 2025 Jul 3;11(1):303. doi: 10.1038/s41420-025-02585-8. Cell Death Discov. 2025. PMID: 40610429 Free PMC article.
-
Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer.Front Immunol. 2022 Oct 20;13:1016817. doi: 10.3389/fimmu.2022.1016817. eCollection 2022. Front Immunol. 2022. PMID: 36341377 Free PMC article. Review.
-
Vascular endothelial growth factor A is a potential prognostic biomarker and correlates with immune cell infiltration in hepatocellular carcinoma.J Cell Mol Med. 2023 Feb;27(4):538-552. doi: 10.1111/jcmm.17678. Epub 2023 Feb 2. J Cell Mol Med. 2023. PMID: 36729917 Free PMC article.
-
LncRNA ABHD11-AS1 Elevates CALM2 to Promote Metastasis of Thyroid Cancer Through Sponging miR-876-5p.Biochem Genet. 2025 Mar 21. doi: 10.1007/s10528-025-11072-9. Online ahead of print. Biochem Genet. 2025. PMID: 40117023
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

