MicroRNAs as Diagnostic Biomarkers in Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis
- PMID: 34604087
- PMCID: PMC8484918
- DOI: 10.3389/fonc.2021.743542
MicroRNAs as Diagnostic Biomarkers in Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis
Abstract
Background: Diagnosing primary central nervous system lymphoma (PCNSL) remains a challenge. MicroRNAs (miRNAs) are promising noninvasive markers for the identification of PCNSL. The present study aims to assess the diagnostic value of miRNAs for PCNSL patients as biomarkers.
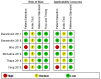
Methods: We systematically searched PubMed, Embase, and the Cochrane library from inception to January 31, 2021. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), together with the summary receiver operator characteristic (SROC) curve, and the area under the SROC curve (AUC) value were used to estimate the overall diagnostic performance. We used Q statistic and I2 to test heterogeneity and used subgroup analyses to investigate the source of heterogeneity. The statistical analyses were independently performed by two investigators using Stata 14.0 and Revman 5.3.
Results: In total, 11 studies from 6 records were included in the current meta-analysis with 281 PCNSL patients and 367 controls. Our statistical analysis demonstrated that the pooled sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.91 (95% CI 0.84-0.95), 0.88 (95% CI 0.84-0.91), 7.48 (95% CI 5.71-9.78), 0.11 (95% CI 0.06-0.19), 70 (95% CI 35-142), and 0.90 (95% CI 0.87-0.92), respectively. The studies had substantial heterogeneity (I2 = 54%, 95% CI 0-100). Two subgroup analyses were conducted based on the type of specimen and miRNAs profiled.
Conclusions: This meta-analysis indicated that miRNAs were suitable as noninvasive diagnostic biomarkers for PCNSL with high accuracy. In addition, both cerebrospinal fluid-based and blood-based miRNAs assays for PCNSL detection were considered reliable for clinical application. MicroRNA-21 assays also seemed to be more accurate in the diagnosis of PCNSL. Good quality studies with large samples should be conducted to verify our results.
Keywords: brain tumor; diagnosis; meta-analysis; microRNAs; primary central nervous system lymphoma.
Copyright © 2021 Zheng, Li, Dong, Duan, Yang, Cai, Chen and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Schorb E, Finke J, Ferreri AJ, Ihorst G, Mikesch K, Kasenda B, et al. High-Dose Chemotherapy and Autologous Stem Cell Transplant Compared With Conventional Chemotherapy for Consolidation in Newly Diagnosed Primary CNS Lymphoma–a Randomized Phase III Trial (MATRix). BMC Cancer (2016) 16:282. doi: 10.1186/s12885-016-2311-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

