Molecular Epidemiology, Antifungal Susceptibility, and Virulence Evaluation of Candida Isolates Causing Invasive Infection in a Tertiary Care Teaching Hospital
- PMID: 34604110
- PMCID: PMC8479822
- DOI: 10.3389/fcimb.2021.721439
Molecular Epidemiology, Antifungal Susceptibility, and Virulence Evaluation of Candida Isolates Causing Invasive Infection in a Tertiary Care Teaching Hospital
Abstract
Background: The incidence of invasive candidiasis is increasing worldwide. However, the epidemiology, antifungal susceptibility, and virulence of Candida spp. in most hospitals remain unclear. This study aimed to evaluate invasive candidiasis in a tertiary care hospital in Nanchang City, China.
Methods: MALDI-TOF MS and 18S rDNA ITS sequencing were used to identify Candida strains. Randomly amplified polymorphic DNA analysis was used for molecular typing; biofilm production, caseinase, and hemolysin activities were used to evaluate virulence. The Sensititre™ YeastOne YO10 panel was used to examine antifungal susceptibility. Mutations in ERG11 and the hotspot regions of FKS1 of drug-resistant strains were sequenced to evaluate the possible mechanisms of antifungal resistance.
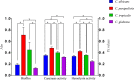
Results: We obtained 110 Candida strains, which included 40 Candida albicans (36.36%), 37 C. parapsilosis (33.64%), 21 C. tropicalis (19.09%), 9 C. glabrata (8.18%), 2 C. rugose (1.82%), and 1 C. haemulonii (0.91%) isolates. At a limiting point of 0.80, C. albicans isolates could be grouped into five clusters, C. parapsilosis and C. tropicalis isolates into seven clusters, and C. glabrata isolates into only one cluster comprising six strains by RAPD typing. Antifungal susceptibility testing revealed that the isolates showed the greatest overall resistance against fluconazole (6.36%), followed by voriconazole (4.55%). All C. albicans and C. parapsilosis isolates exhibited 100% susceptibility to echinocandins (i.e., anidulafungin, caspofungin, and micafungin), whereas one C. glabrata strain was resistant to echinocandins. The most common amino acid substitutions noted in our study was 132aa (Y132H, Y132F) in the azole-resistant strains. No missense mutation was identified in the hotpot regions of FKS1. Comparison of the selected virulence factors detectable in a laboratory environment, such as biofilm, caseinase, and hemolysin production, revealed that most Candida isolates were caseinase and hemolysin producers with a strong activity (Pz < 0.69). Furthermore, C. parapsilosis had greater total biofilm biomass (average Abs620 = 0.712) than C. albicans (average Abs620 = 0.214, p < 0.01) or C. tropicalis (average Abs620 = 0.450, p < 0.05), although all C. glabrata strains were either low- or no-biofilm producers. The virulence level of the isolates from different specimen sources or clusters showed no obvious correlation. Interesting, 75% of the C. albicans from cluster F demonstrated azole resistance, whereas two azole-resistant C. tropicalis strains belonged to the cluster Y.
Conclusion: This study provides vital information regarding the epidemiology, pathogenicity, and antifungal susceptibility of Candida spp. in patients admitted to Nanchang City Hospital.
Keywords: Candida species; RAPD; antifungal susceptibility; biofilm; caseinase; hemolysin.
Copyright © 2021 Chen, Hu, Xu, Liu, Yu, Zhang, Huang, Tan, Huang and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

