Targeting Host Glycolysis as a Strategy for Antimalarial Development
- PMID: 34604112
- PMCID: PMC8482815
- DOI: 10.3389/fcimb.2021.730413
Targeting Host Glycolysis as a Strategy for Antimalarial Development
Abstract
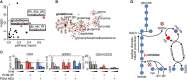
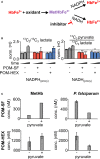
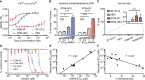
Glycolysis controls cellular energy, redox balance, and biosynthesis. Antiglycolytic therapies are under investigation for treatment of obesity, cancer, aging, autoimmunity, and microbial diseases. Interrupting glycolysis is highly valued as a therapeutic strategy, because glycolytic disruption is generally tolerated in mammals. Unfortunately, anemia is a known dose-limiting side effect of these inhibitors and presents a major caveat to development of antiglycolytic therapies. We developed specific inhibitors of enolase - a critical enzyme in glycolysis - and validated their metabolic and cellular effects on human erythrocytes. Enolase inhibition increases erythrocyte susceptibility to oxidative damage and induces rapid and premature erythrocyte senescence, rather than direct hemolysis. We apply our model of red cell toxicity to address questions regarding erythrocyte glycolytic disruption in the context of Plasmodium falciparum malaria pathogenesis. Our study provides a framework for understanding red blood cell homeostasis under normal and disease states and clarifies the importance of erythrocyte reductive capacity in malaria parasite growth.
Keywords: Plasmodium; antimalarial; enolase; erythrocyte; glycolysis; malaria; oxidative stress; red blood cells.
Copyright © 2021 Jezewski, Lin, Reisz, Culp-Hill, Barekatain, Yan, D’Alessandro, Muller and Odom John.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The Metabolite Repair Enzyme Phosphoglycolate Phosphatase Regulates Central Carbon Metabolism and Fosmidomycin Sensitivity in Plasmodium falciparum.mBio. 2019 Dec 10;10(6):e02060-19. doi: 10.1128/mBio.02060-19. mBio. 2019. PMID: 31822583 Free PMC article.
-
The treatment of Plasmodium falciparum-infected erythrocytes with chloroquine leads to accumulation of ferriprotoporphyrin IX bound to particular parasite proteins and to the inhibition of the parasite's 6-phosphogluconate dehydrogenase.Parasite. 2003 Mar;10(1):39-50. doi: 10.1051/parasite/2003101p39. Parasite. 2003. PMID: 12669348
-
Manipulating Eryptosis of Human Red Blood Cells: A Novel Antimalarial Strategy?Front Cell Infect Microbiol. 2018 Nov 30;8:419. doi: 10.3389/fcimb.2018.00419. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30560094 Free PMC article. Review.
-
A detailed kinetic model of glycolysis in Plasmodium falciparum-infected red blood cells for antimalarial drug target identification.J Biol Chem. 2023 Sep;299(9):105111. doi: 10.1016/j.jbc.2023.105111. Epub 2023 Jul 29. J Biol Chem. 2023. PMID: 37517694 Free PMC article.
-
Parasite and Host Erythrocyte Kinomics of Plasmodium Infection.Trends Parasitol. 2021 Jun;37(6):508-524. doi: 10.1016/j.pt.2021.01.002. Epub 2021 Feb 13. Trends Parasitol. 2021. PMID: 33593681 Review.
Cited by
-
Prodrugs of a 1-Hydroxy-2-oxopiperidin-3-yl Phosphonate Enolase Inhibitor for the Treatment of ENO1-Deleted Cancers.J Med Chem. 2022 Oct 27;65(20):13813-13832. doi: 10.1021/acs.jmedchem.2c01039. Epub 2022 Oct 17. J Med Chem. 2022. PMID: 36251833 Free PMC article.
-
Amino acid supplementation confers protection to red blood cells before Plasmodium falciparum bystander stress.Blood Adv. 2024 May 28;8(10):2552-2564. doi: 10.1182/bloodadvances.2023010820. Blood Adv. 2024. PMID: 38537079 Free PMC article.
-
Inhibition of malaria and babesiosis parasites by putative red blood cell targeting small molecules.Front Cell Infect Microbiol. 2024 Mar 20;14:1304839. doi: 10.3389/fcimb.2024.1304839. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38572319 Free PMC article.
-
In vitro and in silico investigations of Propolis-derived phytochemicals as potential inhibitors of Plasmodium falciparum.Vet World. 2025 Jun;18(6):1644-1659. doi: 10.14202/vetworld.2025.1644-1659. Epub 2025 Jun 19. Vet World. 2025. PMID: 40689184 Free PMC article.
-
Mefloquine-curcumin combinations improve host mitochondrial respiration and decrease mitotoxic effects of mefloquine in Plasmodium berghei-infected mice.Curr Res Pharmacol Drug Discov. 2024 Apr 18;6:100180. doi: 10.1016/j.crphar.2024.100180. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 38725654 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

