Tackling Current Biomedical Challenges With Frontier Biofabrication and Organ-On-A-Chip Technologies
- PMID: 34604190
- PMCID: PMC8481890
- DOI: 10.3389/fbioe.2021.732130
Tackling Current Biomedical Challenges With Frontier Biofabrication and Organ-On-A-Chip Technologies
Abstract
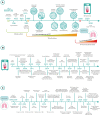
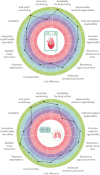
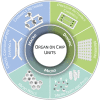
In the last decades, biomedical research has significantly boomed in the academia and industrial sectors, and it is expected to continue to grow at a rapid pace in the future. An in-depth analysis of such growth is not trivial, given the intrinsic multidisciplinary nature of biomedical research. Nevertheless, technological advances are among the main factors which have enabled such progress. In this review, we discuss the contribution of two state-of-the-art technologies-namely biofabrication and organ-on-a-chip-in a selection of biomedical research areas. We start by providing an overview of these technologies and their capacities in fabricating advanced in vitro tissue/organ models. We then analyze their impact on addressing a range of current biomedical challenges. Ultimately, we speculate about their future developments by integrating these technologies with other cutting-edge research fields such as artificial intelligence and big data analysis.
Keywords: 3D biofabrication; drug development; organ-on-a-chip; precision medicine; regenerative medicine; tissue engineering.
Copyright © 2021 Celikkin, Presutti, Maiullari, Fornetti, Agarwal, Paradiso, Volpi, Święszkowski, Bearzi, Barbetta, Zhang, Gargioli, Rizzi and Costantini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

