Neuron-Glia Crosstalk Plays a Major Role in the Neurotoxic Effects of Ketamine via Extracellular Vesicles
- PMID: 34604212
- PMCID: PMC8481868
- DOI: 10.3389/fcell.2021.691648
Neuron-Glia Crosstalk Plays a Major Role in the Neurotoxic Effects of Ketamine via Extracellular Vesicles
Abstract
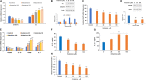
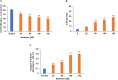
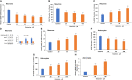
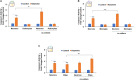
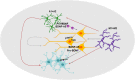
Background: There is a compelling evidence from animal models that early exposure to clinically relevant general anesthetics (GAs) interferes with brain development, resulting in long-lasting cognitive impairments. Human studies have been inconclusive and are challenging due to numerous confounding factors. Here, we employed primary human neural cells to analyze ketamine neurotoxic effects focusing on the role of glial cells and their activation state. We also explored the roles of astrocyte-derived extracellular vesicles (EVs) and different components of the brain-derived neurotrophic factor (BDNF) pathway. Methods: Ketamine effects on cell death were analyzed using live/dead assay, caspase 3 activity and PARP-1 cleavage. Astrocytic and microglial cell differentiation was determined using RT-PCR, ELISA and phagocytosis assay. The impact of the neuron-glial cell interactions in the neurotoxic effects of ketamine was analyzed using transwell cultures. In addition, the role of isolated and secreted EVs in this cross-talk were studied. The expression and function of different components of the BDNF pathway were analyzed using ELISA, RT-PCR and gene silencing. Results: Ketamine induced neuronal and oligodendrocytic cell apoptosis and promoted pro-inflammatory astrocyte (A1) and microglia (M1) phenotypes. Astrocytes and microglia enhanced the neurotoxic effects of ketamine on neuronal cells, whereas neurons increased oligodendrocyte cell death. Ketamine modulated different components in the BDNF pathway: decreasing BDNF secretion in neurons and astrocytes while increasing the expression of p75 in neurons and that of BDNF-AS and pro-BDNF secretion in both neurons and astrocytes. We demonstrated an important role of EVs secreted by ketamine-treated astrocytes in neuronal cell death and a role for EV-associated BDNF-AS in this effect. Conclusions: Ketamine exerted a neurotoxic effect on neural cells by impacting both neuronal and non-neuronal cells. The BDNF pathway and astrocyte-derived EVs represent important mediators of ketamine effects. These results contribute to a better understanding of ketamine neurotoxic effects in humans and to the development of potential approaches to decrease its neurodevelopmental impact.
Keywords: BDNF; BDNF-AS; astrocytes; ketamine; microglia; neurotoxicity.
Copyright © 2021 Penning, Cazacu, Brodie, Jevtovic-Todorovic, Kalkanis, Lewis and Brodie.
Conflict of interest statement
AB was employed by Precise Cell Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
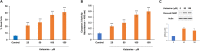

Similar articles
-
Insufficient Astrocyte-Derived Brain-Derived Neurotrophic Factor Contributes to Propofol-Induced Neuron Death Through Akt/Glycogen Synthase Kinase 3β/Mitochondrial Fission Pathway.Anesth Analg. 2017 Jul;125(1):241-254. doi: 10.1213/ANE.0000000000002137. Anesth Analg. 2017. PMID: 28622174 Free PMC article.
-
Necroptotic astrocytes induced neuronal apoptosis partially through EVs-derived pro-BDNF.Brain Res Bull. 2021 Dec;177:73-80. doi: 10.1016/j.brainresbull.2021.09.014. Epub 2021 Sep 20. Brain Res Bull. 2021. PMID: 34555432
-
Pgrmc1/BDNF Signaling Plays a Critical Role in Mediating Glia-Neuron Cross Talk.Endocrinology. 2016 May;157(5):2067-79. doi: 10.1210/en.2015-1610. Epub 2016 Mar 18. Endocrinology. 2016. PMID: 26990062 Free PMC article.
-
Cross-talk signals in the CNS: role of neurotrophic and hormonal factors, adhesion molecules and intercellular signaling agents in luteinizing hormone-releasing hormone (LHRH)-astroglial interactive network.Front Biosci. 1997 Mar 1;2:d88-125. doi: 10.2741/a177. Front Biosci. 1997. PMID: 9159216 Review.
-
Microglia Polarization, Gene-Environment Interactions and Wnt/β-Catenin Signaling: Emerging Roles of Glia-Neuron and Glia-Stem/Neuroprogenitor Crosstalk for Dopaminergic Neurorestoration in Aged Parkinsonian Brain.Front Aging Neurosci. 2018 Feb 12;10:12. doi: 10.3389/fnagi.2018.00012. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29483868 Free PMC article. Review.
Cited by
-
Propofol Inhibits Glioma Stem Cell Growth and Migration and Their Interaction with Microglia via BDNF-AS and Extracellular Vesicles.Cells. 2023 Jul 25;12(15):1921. doi: 10.3390/cells12151921. Cells. 2023. PMID: 37566001 Free PMC article.
-
General anesthetic agents induce neurotoxicity through astrocytes.Neural Regen Res. 2024 Jun 1;19(6):1299-1307. doi: 10.4103/1673-5374.385857. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 37905879 Free PMC article.
-
Nonapoptotic caspases in neural development and in anesthesia-induced neurotoxicity.Trends Neurosci. 2022 Jun;45(6):446-458. doi: 10.1016/j.tins.2022.03.007. Epub 2022 Apr 28. Trends Neurosci. 2022. PMID: 35491256 Free PMC article. Review.
-
Characterization of extracellular vesicles and miRNA released by cerebral organoids.Curr Res Toxicol. 2025 Mar 8;8:100229. doi: 10.1016/j.crtox.2025.100229. eCollection 2025. Curr Res Toxicol. 2025. PMID: 40469891 Free PMC article.
-
Dual roles of anesthetics in postoperative cognitive dysfunction: Regulation of microglial activation through inflammatory signaling pathways.Front Immunol. 2023 Jan 27;14:1102312. doi: 10.3389/fimmu.2023.1102312. eCollection 2023. Front Immunol. 2023. PMID: 36776829 Free PMC article. Review.
References
-
- Baker S. C., Shabir S., Georgopoulos N. T., Southgate J. (2016). Ketamine-Induced apoptosis in normal human urothelial cells: a direct, N-Methyl-d-Aspartate receptor-independent pathway characterized by mitochondrial stress. Am. J. Pathol. 186 1267–1277. 10.1016/j.ajpath.2015.12.014 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

