Reproductive Aging in Caenorhabditis elegans: From Molecules to Ecology
- PMID: 34604218
- PMCID: PMC8481778
- DOI: 10.3389/fcell.2021.718522
Reproductive Aging in Caenorhabditis elegans: From Molecules to Ecology
Abstract
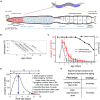
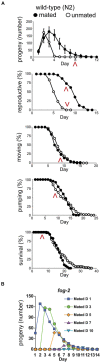
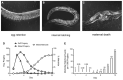
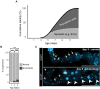
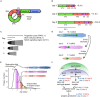
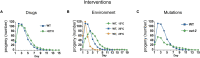
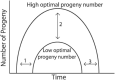
Aging animals display a broad range of progressive degenerative changes, and one of the most fascinating is the decline of female reproductive function. In the model organism Caenorhabditis elegans, hermaphrodites reach a peak of progeny production on day 2 of adulthood and then display a rapid decline; progeny production typically ends by day 8 of adulthood. Since animals typically survive until day 15 of adulthood, there is a substantial post reproductive lifespan. Here we review the molecular and cellular changes that occur during reproductive aging, including reductions in stem cell number and activity, slowing meiotic progression, diminished Notch signaling, and deterioration of germ line and oocyte morphology. Several interventions have been identified that delay reproductive aging, including mutations, drugs and environmental factors such as temperature. The detailed description of reproductive aging coupled with interventions that delay this process have made C. elegans a leading model system to understand the mechanisms that drive reproductive aging. While reproductive aging has dramatic consequences for individual fertility, it also has consequences for the ecology of the population. Population dynamics are driven by birth and death, and reproductive aging is one important factor that influences birth rate. A variety of theories have been advanced to explain why reproductive aging occurs and how it has been sculpted during evolution. Here we summarize these theories and discuss the utility of C. elegans for testing mechanistic and evolutionary models of reproductive aging.
Keywords: aging; egg-laying; evolution; germ line; matricidal hatching; menopause; oocyte quality; reproduction.
Copyright © 2021 Scharf, Pohl, Egan, Kocsisova and Kornfeld.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Age-related degeneration of the egg-laying system promotes matricidal hatching in Caenorhabditis elegans.Aging Cell. 2013 Aug;12(4):544-53. doi: 10.1111/acel.12079. Epub 2013 May 2. Aging Cell. 2013. PMID: 23551912 Free PMC article.
-
Rapid population-wide declines in stem cell number and activity during reproductive aging in C. elegans.Development. 2019 Apr 23;146(8):dev173195. doi: 10.1242/dev.173195. Development. 2019. PMID: 30936182 Free PMC article.
-
Genetic and pharmacological factors that influence reproductive aging in nematodes.PLoS Genet. 2007 Feb 16;3(2):e25. doi: 10.1371/journal.pgen.0030025. Epub 2006 Dec 28. PLoS Genet. 2007. PMID: 17305431 Free PMC article.
-
Caenorhabditis elegans reproductive aging: Regulation and underlying mechanisms.Genesis. 2011 Feb;49(2):53-65. doi: 10.1002/dvg.20694. Epub 2011 Jan 24. Genesis. 2011. PMID: 21105070 Review.
-
Reproductive potential in the older woman.Fertil Steril. 1986 Dec;46(6):989-1001. doi: 10.1016/s0015-0282(16)49869-9. Fertil Steril. 1986. PMID: 3536609 Review.
Cited by
-
Reproductive Span of Caenorhabditis Elegans is Extended by Microbacterium Sp.J Nematol. 2022 May 10;54(1):20220010. doi: 10.2478/jofnem-2022-0010. eCollection 2022 Feb. J Nematol. 2022. PMID: 35860519 Free PMC article.
-
Maternal Gliadin Intake Reduces Oocyte Quality with Chromosomal Aberrations and Increases Embryonic Lethality through Oxidative Stress in a Caenorhabditis elegans Model.Nutrients. 2022 Dec 19;14(24):5403. doi: 10.3390/nu14245403. Nutrients. 2022. PMID: 36558561 Free PMC article.
-
Preconception exposure to perfluorooctanesulfonic acid (PFOS) and perfluorobutanesulfonic acid (PFBS) impaired reproduction via insulin/insulin-like growth factor signaling pathway without effects on the second generation in Caenorhabditis elegans.Toxicol Rep. 2025 Feb 19;14:101966. doi: 10.1016/j.toxrep.2025.101966. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 40066201 Free PMC article.
-
Neuropeptide inactivation regulates egg-laying behavior to influence reproductive health in Caenorhabditis elegans.Curr Biol. 2024 Oct 21;34(20):4715-4728.e4. doi: 10.1016/j.cub.2024.09.059. Epub 2024 Oct 11. Curr Biol. 2024. PMID: 39395417
-
Metabolic analysis of sarcopenic muscle identifies positive modulators of longevity and healthspan in C. elegans.Redox Biol. 2025 Jun 14;85:103732. doi: 10.1016/j.redox.2025.103732. Online ahead of print. Redox Biol. 2025. PMID: 40544604 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

