Combination of 0.05% Azelastine and 0.1% Tacrolimus Eye Drops in Children With Vernal Keratoconjunctivitis: A Prospective Study
- PMID: 34604246
- PMCID: PMC8484704
- DOI: 10.3389/fmed.2021.650083
Combination of 0.05% Azelastine and 0.1% Tacrolimus Eye Drops in Children With Vernal Keratoconjunctivitis: A Prospective Study
Abstract
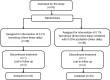
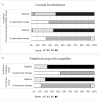
Aims: To compare the efficacy of the combination of 0. 05% azelastine and 0.1% tacrolimus eye drops with 0.1% tacrolimus monotherapy in pediatric patients with vernal keratoconjunctivitis (VKC). Methods: Prospective study. Seventy-six patients with VKC were randomized 1:1 into monotherapy group with 0.1% tacrolimus or combination therapy group with 0.1% tacrolimus and 0.05% azelastine. The Ocular Surface Disease Index (OSDI) scores and the signs of conjunctival hyperemia, corneal involvement, and palpebral conjunctiva papillae were assessed at baseline and at 1, 2, and 6 weeks after treatment. Results: Two groups were comparable in age, sex, duration of VKC, OSDI, and clinical signs of VKC at baseline. Significant improvements in OSDI score and clinical signs were observed in both groups at all follow-up visits (all p < 0.001), compared with baseline. The combination therapy group showed a larger decrease in OSDI score from baseline (10.30 ± 0.9) compared with monotherapy group (7.30 ± 0.7, p =0.0085) at 1 week. Greater improvements in conjunctival hyperemia and conjunctival papillae were identified in the combination therapy group, compared with in the monotherapy group, at all follow-up visits (all p < 0.05). The corneal involvement scores in the combination group is significantly lower than the monotherapy group at 2 weeks after the treatment (p = 0.0488). No severe adverse effect was found in either group during the study. Conclusions: Compared with a monotherapy of 0.1% tacrolimus, the combination of 0.05% azelastine and 0.1% tacrolimus eye drops lead to faster and greater improvements in clinical signs and symptoms of vernal keratoconjunctivitis in pediatric patients.
Keywords: azelastine; ocular surface disease index; palpebral conjunctival papillae; tacrolimus; vernal conjunctivitis.
Copyright © 2021 Chen, Wei, Ke, Zou, Gong, Wang, Zhang, Xu, Yin and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Medical

