Multifrequency STD NMR Unveils the Interactions of Antibiotics With Burkholderia multivorans Biofilm Exopolysaccharide
- PMID: 34604306
- PMCID: PMC8481691
- DOI: 10.3389/fmolb.2021.727980
Multifrequency STD NMR Unveils the Interactions of Antibiotics With Burkholderia multivorans Biofilm Exopolysaccharide
Abstract
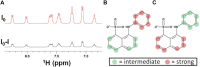
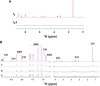
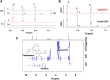
Biofilms confine bacterial cells within self-produced matrices, offering advantages such as protection from antibiotics and entrapment of nutrients. Polysaccharides are major components in these macromolecular assemblies, and their interactions with other chemicals are of high relevance for the benefits provided by the biofilm 3D molecular matrix. NMR is a powerful technique for the study and characterization of the interactions between molecules of biological relevance. In this study, we have applied multifrequency saturation transfer difference (STD) NMR and DOSY NMR approaches to elucidate the interactions between the exopolysaccharide produced by Burkholderia multivorans C1576 (EpolC1576) and the antibiotics kanamycin and ceftadizime. The NMR strategies presented here allowed for an extensive characterization at an atomic level of the mechanisms behind the implication of the EpolC1576 in the recalcitrance phenomena, which is the ability of bacteria in biofilms to survive in the presence of antibiotics. Our results suggest an active role for EpolC1576 in the recalcitrance mechanisms toward kanamycin and ceftadizime, though through two different mechanisms.
Keywords: Burkholderia multivorans; STD NMR; biofilms; exopolysaccharides; multifrequency STD NMR.
Copyright © 2021 Nepravishta, Monaco, Distefano, Rizzo, Cescutti and Angulo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
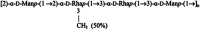


Similar articles
-
A novel rhamno-mannan exopolysaccharide isolated from biofilms of Burkholderia multivorans C1576.Carbohydr Res. 2015 Jun 26;411:42-8. doi: 10.1016/j.carres.2015.04.012. Epub 2015 Apr 28. Carbohydr Res. 2015. PMID: 25974852
-
Fluorescence and NMR spectroscopy together with molecular simulations reveal amphiphilic characteristics of a Burkholderia biofilm exopolysaccharide.J Biol Chem. 2017 Jun 30;292(26):11034-11042. doi: 10.1074/jbc.M117.785048. Epub 2017 May 3. J Biol Chem. 2017. PMID: 28468829 Free PMC article.
-
Microscopy and modelling investigations on the morphology of the biofilm exopolysaccharide produced by Burkholderia multivorans strain C1576.Int J Biol Macromol. 2023 Dec 31;253(Pt 6):127294. doi: 10.1016/j.ijbiomac.2023.127294. Epub 2023 Oct 7. Int J Biol Macromol. 2023. PMID: 37813217 Free PMC article.
-
Regulation of Biofilm Exopolysaccharide Biosynthesis and Degradation in Pseudomonas aeruginosa.Annu Rev Microbiol. 2022 Sep 8;76:413-433. doi: 10.1146/annurev-micro-041320-111355. Epub 2022 Jun 2. Annu Rev Microbiol. 2022. PMID: 35655342 Review.
-
The Role of Exopolysaccharides in Oral Biofilms.J Dent Res. 2019 Jul;98(7):739-745. doi: 10.1177/0022034519845001. Epub 2019 Apr 22. J Dent Res. 2019. PMID: 31009580 Free PMC article. Review.
Cited by
-
Glycosaminoglycans: What Remains To Be Deciphered?JACS Au. 2023 Mar 2;3(3):628-656. doi: 10.1021/jacsau.2c00569. eCollection 2023 Mar 27. JACS Au. 2023. PMID: 37006755 Free PMC article. Review.
-
Fast Quantitative Validation of 3D Models of Low-Affinity Protein-Ligand Complexes by STD NMR Spectroscopy.J Med Chem. 2024 Jun 27;67(12):10025-10034. doi: 10.1021/acs.jmedchem.4c00204. Epub 2024 Jun 7. J Med Chem. 2024. PMID: 38848103 Free PMC article.
-
The Molecular Mechanism of FABP4 Inhibition Effects of GAS and 4-HBA in Gastrodia elata Blume Was Discussed Based on NMR and Molecular Docking.J Anal Methods Chem. 2024 May 8;2024:6599029. doi: 10.1155/2024/6599029. eCollection 2024. J Anal Methods Chem. 2024. PMID: 38751858 Free PMC article.
References
-
- Airoldi C., Giovannardi S., La Ferla B., Jiménez-Barbero J., Nicotra F. (2011). Saturation Transfer Difference NMR Experiments of Membrane Proteins in Living Cells under HR-MAS Conditions: the Interaction of the SGLT1 Co-transporter with its Ligands. Chem. Eur. J. 17, 13395–13399. 10.1002/chem.201102181 - DOI - PubMed
-
- Calabrese V., Muñoz-García J. C., Schmitt J., da Silva M. A., Scott J. L., Angulo J., et al. (2019). Understanding Heat Driven Gelation of Anionic Cellulose Nanofibrils: Combining Saturation Transfer Difference (STD) NMR, Small Angle X-ray Scattering (SAXS) and Rheology. J. Colloid Interf. Sci. 535, 205–213. 10.1016/j.jcis.2018.09.085 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

