Next-Generation Intestinal Toxicity Model of Human Embryonic Stem Cell-Derived Enterocyte-Like Cells
- PMID: 34604364
- PMCID: PMC8481684
- DOI: 10.3389/fvets.2021.587659
Next-Generation Intestinal Toxicity Model of Human Embryonic Stem Cell-Derived Enterocyte-Like Cells
Abstract
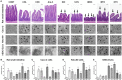
The gastrointestinal tract is the most common exposure route of xenobiotics, and intestinal toxicity can result in systemic toxicity in most cases. It is important to develop intestinal toxicity assays mimicking the human system; thus, stem cells are rapidly being developed as new paradigms of toxicity assessment. In this study, we established human embryonic stem cell (hESC)-derived enterocyte-like cells (ELCs) and compared them to existing in vivo and in vitro models. We found that hESC-ELCs and the in vivo model showed transcriptomically similar expression patterns of a total of 10,020 genes than the commercialized cell lines. Besides, we treated the hESC-ELCs, in vivo rats, Caco-2 cells, and Hutu-80 cells with quarter log units of lethal dose 50 or lethal concentration 50 of eight drugs-chloramphenicol, cycloheximide, cytarabine, diclofenac, fluorouracil, indomethacin, methotrexate, and oxytetracycline-and then subsequently analyzed the biomolecular markers and morphological changes. While the four models showed similar tendencies in general toxicological reaction, hESC-ELCs showed a stronger correlation with the in vivo model than the immortalized cell lines. These results indicate that hESC-ELCs can serve as a next-generation intestinal toxicity model.
Keywords: alternative testing method; embryonic stem cell; enterotoxicity; intestinal toxicity; toxicology.
Copyright © 2021 Ryu, Son, Jung, Kim, Kim, Kwon, Son, Jung, Park and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Development of an alternative zebrafish model for drug-induced intestinal toxicity.J Appl Toxicol. 2018 Feb;38(2):259-273. doi: 10.1002/jat.3520. Epub 2017 Oct 13. J Appl Toxicol. 2018. PMID: 29027214
-
Modeling of drug-mediated CYP3A4 induction by using human iPS cell-derived enterocyte-like cells.Biochem Biophys Res Commun. 2016 Apr 15;472(4):631-6. doi: 10.1016/j.bbrc.2016.03.012. Epub 2016 Mar 8. Biochem Biophys Res Commun. 2016. PMID: 26966071
-
Generation of enterocyte-like cells from human induced pluripotent stem cells for drug absorption and metabolism studies in human small intestine.Sci Rep. 2015 Nov 12;5:16479. doi: 10.1038/srep16479. Sci Rep. 2015. PMID: 26559489 Free PMC article.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Stem cell models for drug discovery and toxicology studies.J Biochem Mol Toxicol. 2013 Jan;27(1):17-27. doi: 10.1002/jbt.21470. Epub 2013 Jan 4. J Biochem Mol Toxicol. 2013. PMID: 23293059 Review.
Cited by
-
NSAID-Associated Small Intestinal Injury: An Overview From Animal Model Development to Pathogenesis, Treatment, and Prevention.Front Pharmacol. 2022 Feb 9;13:818877. doi: 10.3389/fphar.2022.818877. eCollection 2022. Front Pharmacol. 2022. PMID: 35222032 Free PMC article. Review.
-
A method to determine reactive oxygen species production in intestinal and liver cell cultures using the 2',7'-dichlorodihydrofluorescein diacetate assay.MethodsX. 2024 Feb 13;12:102615. doi: 10.1016/j.mex.2024.102615. eCollection 2024 Jun. MethodsX. 2024. PMID: 38379720 Free PMC article.
-
Quantification of Pesticides and In Vitro Effects of Water-Soluble Fractions of Agricultural Soils in South Africa.Arch Environ Contam Toxicol. 2025 Feb;88(2):230-250. doi: 10.1007/s00244-025-01115-y. Epub 2025 Feb 15. Arch Environ Contam Toxicol. 2025. PMID: 39955400 Free PMC article.
References
LinkOut - more resources
Full Text Sources

