Molecular Profiling of Spermatozoa Reveals Correlations between Morphology and Gene Expression: A Novel Biomarker Panel for Male Infertility
- PMID: 34604380
- PMCID: PMC8485144
- DOI: 10.1155/2021/1434546
Molecular Profiling of Spermatozoa Reveals Correlations between Morphology and Gene Expression: A Novel Biomarker Panel for Male Infertility
Abstract
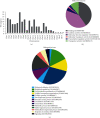
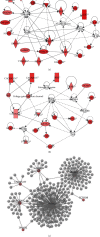
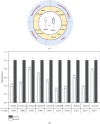
Choosing spermatozoa with an optimum fertilizing potential is one of the major challenges in assisted reproductive technologies (ART). This selection is mainly based on semen parameters, but the addition of molecular approaches could allow a more functional evaluation. To this aim, we used sixteen fresh sperm samples from patients undergoing ART for male infertility and classified them in the high- and poor-quality groups, on the basis of their morphology at high magnification. Then, using a DNA sequencing method, we analyzed the spermatozoa methylome to identify genes that were differentially methylated. By Gene Ontology and protein-protein interaction network analyses, we defined candidate genes mainly implicated in cell motility, calcium reabsorption, and signaling pathways as well as transmembrane transport. RT-qPCR of high- and poor-quality sperm samples allowed showing that the expression of some genes, such as AURKA, HDAC4, CFAP46, SPATA18, CACNA1C, CACNA1H, CARHSP1, CCDC60, DNAH2, and CDC88B, have different expression levels according to sperm morphology. In conclusion, the present study shows a strong correlation between morphology and gene expression in the spermatozoa and provides a biomarker panel for sperm analysis during ART and a new tool to explore male infertility.
Copyright © 2021 Nino Guy Cassuto et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Poongothai J., Gopenath T. S., Manonayaki S. Genetics of human male infertility. Singapore Medical Journal . 2009;50(4):336–347. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

