New 1,2,3-Triazole-Appended Bis-pyrazoles: Synthesis, Bioevaluation, and Molecular Docking
- PMID: 34604669
- PMCID: PMC8482464
- DOI: 10.1021/acsomega.1c03734
New 1,2,3-Triazole-Appended Bis-pyrazoles: Synthesis, Bioevaluation, and Molecular Docking
Abstract
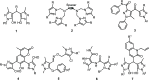
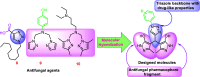
The present work describes design of a small library of new 1,2,3-triazole-appended bis-pyrazoles by using a molecular hybridization approach, and the synthesized hybrids were evaluated for their antifungal activity against different fungal strains, namely, Candida albicans, Cryptococcus neoformans, Candida glabrata, Candida tropicalis, Aspergillus niger, and Aspergillus fumigatus. All the compounds exhibited broad-spectrum activity against the tested fungal strains with excellent minimum inhibitory concentration values. The molecular docking study against sterol 14α-demethylase (CYP51) could provide valuable insights into the binding modes and affinity of these compounds. Furthermore, these compounds were also evaluated for their antioxidant activity, which also resulted in promising data.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
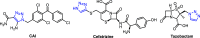





Similar articles
-
Quinoline Based Monocarbonyl Curcumin Analogs as Potential Antifungal and Antioxidant Agents: Synthesis, Bioevaluation and Molecular Docking Study.Chem Biodivers. 2020 Feb;17(2):e1900624. doi: 10.1002/cbdv.201900624. Epub 2020 Jan 22. Chem Biodivers. 2020. PMID: 31863703
-
New N-phenylacetamide-linked 1,2,3-triazole-tethered coumarin conjugates: Synthesis, bioevaluation, and molecular docking study.Arch Pharm (Weinheim). 2020 Nov;353(11):e2000164. doi: 10.1002/ardp.202000164. Epub 2020 Aug 9. Arch Pharm (Weinheim). 2020. PMID: 32776355
-
Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity.Antimicrob Agents Chemother. 2017 Jun 27;61(7):e00570-17. doi: 10.1128/AAC.00570-17. Print 2017 Jul. Antimicrob Agents Chemother. 2017. PMID: 28461309 Free PMC article.
-
Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones.Molecules. 2016 May 10;21(5):484. doi: 10.3390/molecules21050484. Molecules. 2016. PMID: 27171073 Free PMC article.
-
Design, synthesis, and structure-activity relationship studies of novel triazole agents with strong antifungal activity against Aspergillus fumigatus.Bioorg Med Chem Lett. 2020 Feb 15;30(4):126951. doi: 10.1016/j.bmcl.2020.126951. Epub 2020 Jan 7. Bioorg Med Chem Lett. 2020. PMID: 31926784
Cited by
-
Determining the optimal antioxidant strategy: a comparative analysis of extraction methods in Cistus villosus L. extracts revealed by LC-HRMS and computational modeling.Turk J Biol. 2024 Nov 12;49(1):1-27. doi: 10.55730/1300-0152.2720. eCollection 2025. Turk J Biol. 2024. PMID: 40104574 Free PMC article.
-
The Spectroscopic Characterization and Photophysical Properties of a Hydrated Lanthanum Ion Complex with a Triazole Ligand by Several DFT Methods.Molecules. 2025 Aug 18;30(16):3412. doi: 10.3390/molecules30163412. Molecules. 2025. PMID: 40871565 Free PMC article.
-
Exploring the synthetic potential of a g-C3N4·SO3H ionic liquid catalyst for one-pot synthesis of 1,1-dihomoarylmethane scaffolds via Knoevenagel-Michael reaction.RSC Adv. 2023 May 2;13(19):13337-13353. doi: 10.1039/d3ra01971c. eCollection 2023 Apr 24. RSC Adv. 2023. PMID: 37143699 Free PMC article.
-
Synthesis of Benzimidazole-1,2,4-triazole Derivatives as Potential Antifungal Agents Targeting 14α-Demethylase.ACS Omega. 2023 Jan 19;8(4):4369-4384. doi: 10.1021/acsomega.2c07755. eCollection 2023 Jan 31. ACS Omega. 2023. PMID: 36743066 Free PMC article.
-
Synthesis, In Silico Study, and In Vitro Antifungal Activity of New 5-(1,3-Diphenyl-1H-Pyrazol-4-yl)-4-Tosyl-4,5-Dihydrooxazoles.Int J Mol Sci. 2024 May 7;25(10):5091. doi: 10.3390/ijms25105091. Int J Mol Sci. 2024. PMID: 38791130 Free PMC article.
References
-
- Manetti F.; Castagnolo D.; Raffi F.; Zizzari A. T.; Rajamäki S.; D’Arezzo S.; Visca P.; Cona A.; Fracasso M. E.; Doria D.; Posteraro B.; Sanguinetti M.; Fadda G.; Botta M. Synthesis of New Linear Guanidines and Macrocyclic Amidinourea Derivatives Endowed with High Antifungal Activity against Candida spp. and Aspergillus spp. J. Med. Chem. 2009, 52, 7376–7379. 10.1021/jm900760k. - DOI - PubMed
-
- Karrouchi K.; Radi S.; Ramli Y.; Taoufik J.; Mabkhot Y.; Al-aizari F.; Ansar M. h. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134.10.3390/molecules23010134. - DOI - PMC - PubMed
- Keter F. K.; Darkwa J. Perspective: the potential of pyrazole-based compounds in medicine. BioMetals 2012, 25, 9–21. 10.1007/s10534-011-9496-4. - DOI - PubMed
- Naim M. J.; Alam O.; Nawaz F.; Alam M. J.; Alam P. Current status of pyrazole and its biological activities. J. Pharm. BioAllied Sci. 2016, 8, 2–17. 10.4103/0975-7406.171694. - DOI - PMC - PubMed
- Alam M. J.; Alam O.; Alam P.; Naim M. J. A Review on pyrazole chemical entity and biological activity. Int. J. Pharma Sci. Res. 2015, 6, 1433–1442.
LinkOut - more resources
Full Text Sources
Miscellaneous

