Avelumab in newly diagnosed glioblastoma
- PMID: 34604752
- PMCID: PMC8482788
- DOI: 10.1093/noajnl/vdab118
Avelumab in newly diagnosed glioblastoma
Abstract
Background: Glioblastoma (GBM) is known to use both local and systemic immunosuppressive strategies. One such strategy is the expression of the immune checkpoint protein programmed cell death ligand-1 (PD-L1) by both tumor cells and tumor-associated immune cells. Recent phase III trials using IgG4 antibodies targeting PD-1, the ligand for PD-L1, failed to show any benefit. Avelumab is an IgG1 monoclonal antibody targeting PD-L1. In contrast to the previously tested immune checkpoint inhibitors, it can directly bind tumor cells and immune cells expressing PD-L1 and can induce antibody-dependent cellular cytotoxicity.
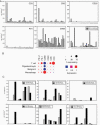
Methods: We conducted a single center, open label, phase II study where avelumab 10 mg/kg IV Q2W was added concurrently to the first monthly temozolomide cycle in patients with newly diagnosed GBM. Immunohistochemical analyses were performed on surgery samples. The primary objective was safety. Secondary objectives were efficacy outcomes according to the immunotherapy Response Assessment in Neuro Oncology criteria, progression free survival (PFS), and overall survival (OS). Exploratory objectives aimed at determining prognostic biomarkers.
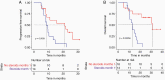
Results: Thirty patients were started on therapy and two were lost to follow-up. Median follow-up time (reverse Kaplan-Meier) was 41.7 months (IQR: 28.3-43.4). Three (10.0%) patients had a related or possibly related treatment emergent adverse event that lead to transient or permanent discontinuation of avelumab. Eight (26.7%) patients had one or more immune-related adverse events, and 8 (26.7%) patients had an infusion-related reaction. The overall response rate was 23.3%, median PFS was 9.7 months, and the median OS was 15.3 months. No pretreatment biomarkers showed any predictive value.
Conclusions: The addition of avelumab to standard therapy in patients with GBM was not associated with any new safety signal. There was no apparent improvement in OS.
Trial registration: NCT03047473 Registered February 9, 2017.
Keywords: PD-L1; avelumab; glioblastoma; immune checkpoint inhibitor; phase II.
© The Author(s) 2021. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Preusser M, de Ribaupierre S, Wöhrer A, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. - PubMed
-
- Central Brain Tumor Registry of the United States. http://www.cbtrus.org/. Accessed December 8, 2020.
-
- Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. - PubMed
-
- Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
