Intradermal-delivered DNA vaccine induces durable immunity mediating a reduction in viral load in a rhesus macaque SARS-CoV-2 challenge model
- PMID: 34604818
- PMCID: PMC8479327
- DOI: 10.1016/j.xcrm.2021.100420
Intradermal-delivered DNA vaccine induces durable immunity mediating a reduction in viral load in a rhesus macaque SARS-CoV-2 challenge model
Abstract
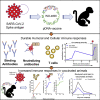
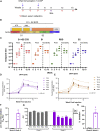
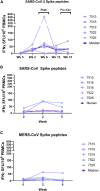
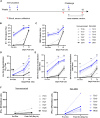
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, has had a dramatic global impact on public health and social and economic infrastructures. Here, we assess the immunogenicity and anamnestic protective efficacy in rhesus macaques of an intradermal (i.d.)-delivered SARS-CoV-2 spike DNA vaccine, INO-4800, currently being evaluated in clinical trials. Vaccination with INO-4800 induced T cell responses and induced spike antigen and RBD binding antibodies with ADCP and ADCD activity. Sera from the animals neutralized both the D614 and G614 SARS-CoV-2 pseudotype viruses. Several months after vaccination, animals were challenged with SARS-CoV-2 resulting in rapid recall of anti-SARS-CoV-2 spike protein T cell and neutralizing antibody responses. These responses were associated with lower viral loads in the lung. These studies support the immune impact of INO-4800 for inducing both humoral and cellular arms of the adaptive immune system, which are likely important for providing durable protection against COVID-19 disease.
Keywords: COVID-19; ID DNA vaccine; SARS-CoV-2; challenge; coronavirus; electroporation; infectious disease; macaque; protection.
© 2021 The Author(s).
Conflict of interest statement
A.P., E.L.R., E.P., E.N.G., M.P., S.N.W., P.B., Z.X., S.M.R., K.Y.K., N.C., E.T-R., J.C., N.J.T., K.M., and D.K.W. declare no competing interests. J.N.W., K.S., I.M., Z.E., D.G., A.D., D.E., A.G., V.M.A., J.J.K., L.M.H., S.J.R., T.R.F.S., and K.E.B. are employees of Inovio Pharmaceuticals and as such receive salary and benefits, including ownership of stock and stock options, from the company. D.B.W. discloses the following paid associations with commercial partners: GeneOne (Consultant), Geneos (Advisory Board), Astrazeneca (Advisory Board, Speaker), Inovio (BOD, SRA, Stock), Pfizer (Speaker), Merck (Speaker), Sanofi (Advisory Board), BBI (Advisory Board).
Figures

References
-
- Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. - PubMed
-
- FDA . US Department of Health and Human Services, Food and Drug Administration; 2020. COVID-19 Vaccines.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

