Selection between Competing Self-Reproducing Lipids: Succession and Dynamic Activation
- PMID: 34604845
- PMCID: PMC8479773
- DOI: 10.1021/jacsau.1c00138
Selection between Competing Self-Reproducing Lipids: Succession and Dynamic Activation
Abstract
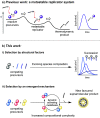
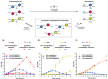
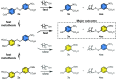
Models of chemical evolution are central to advancing origins of life research. To design more lifelike systems, we must expand our understanding of molecular selection mechanisms. Here, we show two selection modes that produce evolving populations of self-reproducing species, formed through thiol-disulfide exchange. Competition between thiol precursors can give clear succession patterns based on steric factors, an intrinsic property. A separate, emergent selection mechanism-dynamic activating metathesis-was found when exploring competing disulfide precursors. These experiments reveal that additional species generated in the mixture open up alternative reaction pathways to form self-reproducing products. Thus, increased compositional complexity provides certain species with a unique competitive advantage at the expense of others.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Understanding the Early Biological Effects of Isoprene-Derived Particulate Matter Enhanced by Anthropogenic Pollutants.Res Rep Health Eff Inst. 2019 Mar;2019(198):1-54. Res Rep Health Eff Inst. 2019. PMID: 31872748 Free PMC article.
-
Protein thiol-disulfide interchange and interfacing with biological systems.Adv Exp Med Biol. 1977;86A:43-50. doi: 10.1007/978-1-4684-3282-4_3. Adv Exp Med Biol. 1977. PMID: 335840 Review.
-
Thiol-disulfide exchange in signaling: disulfide bonds as a switch.Antioxid Redox Signal. 2013 May 1;18(13):1594-6. doi: 10.1089/ars.2012.5156. Epub 2013 Feb 25. Antioxid Redox Signal. 2013. PMID: 23330837
-
Parasitic behavior in competing chemically fueled reaction cycles.Chem Sci. 2021 Apr 28;12(21):7554-7560. doi: 10.1039/d1sc01106e. Chem Sci. 2021. PMID: 34163846 Free PMC article.
-
Self-reproducing catalytic micelles as nanoscopic protocell precursors.Nat Rev Chem. 2021 Dec;5(12):870-878. doi: 10.1038/s41570-021-00329-7. Epub 2021 Oct 20. Nat Rev Chem. 2021. PMID: 37117387 Review.
Cited by
-
Amplification of Asymmetry via Structural Transitions in Supramolecular Polymer-Surfactant Coassemblies.J Am Chem Soc. 2025 May 21;147(20):17468-17476. doi: 10.1021/jacs.5c04047. Epub 2025 May 8. J Am Chem Soc. 2025. PMID: 40338208 Free PMC article.
-
Anion-assisted amidinium exchange and metathesis.Chem Commun (Camb). 2022 Sep 13;58(73):10178-10181. doi: 10.1039/d2cc03425e. Chem Commun (Camb). 2022. PMID: 35997205 Free PMC article.
-
A Chemical Reaction Network Drives Complex Population Dynamics in Oscillating Self-Reproducing Vesicles.J Am Chem Soc. 2024 Jul 10;146(27):18262-18269. doi: 10.1021/jacs.4c00860. Epub 2024 Jun 25. J Am Chem Soc. 2024. PMID: 38917079 Free PMC article.
-
From autocatalysis to survival of the fittest in self-reproducing lipid systems.Nat Rev Chem. 2023 Oct;7(10):673-691. doi: 10.1038/s41570-023-00524-8. Epub 2023 Aug 23. Nat Rev Chem. 2023. PMID: 37612460 Review.
-
Transient self-assembly of metal-organic complexes.Chem Sci. 2023 Jan 9;14(5):1244-1251. doi: 10.1039/d2sc06374c. eCollection 2023 Feb 1. Chem Sci. 2023. PMID: 36756320 Free PMC article.
References
-
- Luisi P. L.The Emergence of Life: From Chemical Origins to Synthetic Biology; Cambridge University Press: Cambridge, 2006; Vol. 1.10.1017/CBO9780511817540. - DOI
-
- Islam S.; Bučar D. K.; Powner M. W. Prebiotic Selection and Assembly of Proteinogenic Amino Acids and Natural Nucleotides from Complex Mixtures. Nat. Chem. 2017, 9 (6), 584–589. 10.1038/nchem.2703. - DOI
LinkOut - more resources
Full Text Sources
