Biosynthetic Crossover of 5-Lipoxygenase and Cyclooxygenase-2 Yields 5-Hydroxy-PGE2 and 5-Hydroxy-PGD2
- PMID: 34604848
- PMCID: PMC8479768
- DOI: 10.1021/jacsau.1c00177
Biosynthetic Crossover of 5-Lipoxygenase and Cyclooxygenase-2 Yields 5-Hydroxy-PGE2 and 5-Hydroxy-PGD2
Abstract
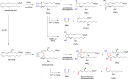
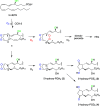
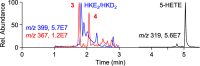
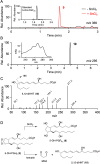
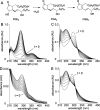
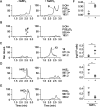
The biosynthetic crossover of 5-lipoxygenase (5-LOX) and cyclooxygenase-2 (COX-2) enzymatic activities is a productive pathway to convert arachidonic acid into unique eicosanoids. Here, we show that COX-2 catalysis with 5-LOX derived 5-hydroxy-eicosatetraenoic acid yields the endoperoxide 5-hydroxy-PGH2 that spontaneously rearranges to 5-OH-PGE2 and 5-OH-PGD2, the 5-hydroxy analogs of arachidonic acid derived PGE2 and PGD2. The endoperoxide was identified via its predicted degradation product, 5,12-dihydroxy-heptadecatri-6E,8E,10E-enoic acid, and by SnCl2-mediated reduction to 5-OH-PGF2α. Both 5-OH-PGE2 and 5-OH-PGD2 were unstable and degraded rapidly upon treatment with weak base. This instability hampered detection in biologic samples which was overcome by in situ reduction using NaBH4 to yield the corresponding stable 5-OH-PGF2 diastereomers and enabled detection of 5-OH-PGF2α in activated primary human leukocytes. 5-OH-PGE2 and 5-OH-PGD2 were unable to activate EP and DP prostanoid receptors, suggesting their bioactivity is distinct from PGE2 and PGD2.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity.Circ Res. 1998 Aug 24;83(4):353-65. doi: 10.1161/01.res.83.4.353. Circ Res. 1998. PMID: 9721692
-
Arachidonic acid metabolism in guinea pig skin: effects of chloroquine.Agents Actions. 1982 Oct;12(4):527-9. doi: 10.1007/BF01965938. Agents Actions. 1982. PMID: 6817622
-
Characterization of 11-HETE and 15-HETE, together with prostacyclin, as major products of the cyclooxygenase pathway in cultured rat aorta smooth muscle cells.J Lipid Res. 1983 Nov;24(11):1419-28. J Lipid Res. 1983. PMID: 6418841
-
The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor.Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Prog Lipid Res. 2013. PMID: 24056189 Free PMC article. Review.
-
Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System.Mol Neurobiol. 2016 Sep;53(7):4754-71. doi: 10.1007/s12035-015-9355-3. Epub 2015 Sep 2. Mol Neurobiol. 2016. PMID: 26328537 Review.
Cited by
-
Modifiable Innate Biology within the Gut-Brain Axis for Alzheimer's Disease.Biomedicines. 2022 Aug 27;10(9):2098. doi: 10.3390/biomedicines10092098. Biomedicines. 2022. PMID: 36140198 Free PMC article. Review.
-
The Link between Prostanoids and Cardiovascular Diseases.Int J Mol Sci. 2023 Feb 20;24(4):4193. doi: 10.3390/ijms24044193. Int J Mol Sci. 2023. PMID: 36835616 Free PMC article. Review.
-
A helping HAND: therapeutic potential of MAGL inhibition against HIV-1-associated neuroinflammation.Front Immunol. 2024 May 21;15:1374301. doi: 10.3389/fimmu.2024.1374301. eCollection 2024. Front Immunol. 2024. PMID: 38835765 Free PMC article.
-
Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review.Int J Mol Sci. 2021 Jul 25;22(15):7937. doi: 10.3390/ijms22157937. Int J Mol Sci. 2021. PMID: 34360703 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
