BMS-986263 in patients with advanced hepatic fibrosis: 36-week results from a randomized, placebo-controlled phase 2 trial
- PMID: 34605045
- PMCID: PMC9299674
- DOI: 10.1002/hep.32181
BMS-986263 in patients with advanced hepatic fibrosis: 36-week results from a randomized, placebo-controlled phase 2 trial
Abstract
Background and aims: Hepatic fibrosis secondary to HCV infection can lead to cirrhosis and hepatic decompensation. Sustained virologic response (SVR) is possible with direct-acting antiviral drug regimens; however, patients with advanced fibrosis have an increased risk for HCC. Heat shock protein 47 (HSP47), a key collagen chaperone, has been implicated in fibrosis development. We evaluated the efficacy and safety of BMS-986263, a lipid nanoparticle delivering small interfering RNA designed to degrade HSP47 mRNA, for the treatment of advanced fibrosis.
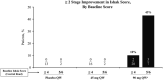
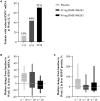
Approach and results: NCT03420768 was a Phase 2, randomized (1:1:2), placebo-controlled trial conducted at a hepatology clinic in the United States. Patients with HCV-SVR (for ≥ 1 year) and advanced fibrosis received once-weekly i.v. infusions of placebo or BMS-986263 (45 or 90 mg) for 12 weeks. The primary endpoint was ≥ 1 METAVIR stage improvement at Week 12; key secondary endpoints included Ishak score improvement, pharmacokinetics, fibrosis biomarkers, and safety. All 61 patients completed treatment, and 2/15 (13%, placebo), 3/18 (17%, 45 mg), and 6/28 (21%, 90 mg) had METAVIR improvements of ≥ 1 stage at Week 12. Five patients in the 90-mg arm had Ishak improvements by ≥ 2 stages. BMS-986263 plasma concentrations increased in a generally dose-proportional fashion between BMS-986263 doses, with no notable accumulation with weekly dosing. All adverse events (AEs) were mild or moderate in intensity; most treatment-related AEs were infusion-related reactions in the BMS-986263 arms. At baseline, collagen levels were low, indicating low levels of fibrogenesis in these patients.
Conclusions: In patients with HCV-SVR, BMS-986263 administration was generally well tolerated through Week 36 and resulted in METAVIR and Ishak score improvements. Further evaluation of BMS-986263 in patients with active fibrogenesis is warranted.
© 2021 Bristol Myers Squibb. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Dr. Lawitz consults for and received grants from Metacrine, Boehringer Ingelheim, and Bristol Myers Squibb. He received grants from 89Bio, AbbVie, Akcea, Arena, Celgene, Conatus, Durect, Enanta, Enyo, Galectin, Galmed, Genfit, Gilead, Hanmi, Intercept, Madrigal, Novartis, Novo Nordisk, Octeta, and Zydus. Dr. Shevell was an employee of Bristol Myers Squibb when the study was performed and owns company stock. Dr. Tirucherai is employed by, owns stock in, and holds intellectual property rights with Bristol Myers Squibb. Dr. Du is employed by and owns stock in Bristol Myers Squibb. Dr. Karsdal is employed by and owns stock in Nordic Bioscience. Dr. Nielsen is employed by and owns stock in Nordic Bioscience. M. Karsdal and M. Nielsen are among the original inventors and patent holders of assays to measure PRO‐C3 and C3M. Dr. Charles is employed by, owns stock in, and holds intellectual property rights with Bristol Myers Squibb. W. Chen was an employee of Bristol Myers Squibb at the time of this study and may hold company stock. U. Kavita was an employee of Bristol Myers Squibb at the time of this study and may hold company stock. A. Coste has no disclosures to report. F. Poordad has participated in speakers’ bureaus for and received grants from Gilead Sciences and Intercept Pharmaceuticals. Z. Goodman has received grants from Alexion Pharmaceuticals, Allergan, Conatus Pharmaceuticals, Exalenz Bioscience, Galactin Therapeutics, Gilead Sciences, and Intercept Pharmaceuticals.
Figures



References
-
- Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology. 2018;155:443–57.e17. - PubMed
-
- Xu F, Moorman AC, Tong X, Gordon SC, Rupp LB, Lu M, et al. All‐cause mortality and progression risks to hepatic decompensation and hepatocellular carcinoma in patients infected with hepatitis C virus. Clin Infect Dis. 2016;62:289–97. - PubMed
-
- Curry MP, Tapper EB, Bacon B, Dieterich D, Flamm SL, Guest L, et al. Effectiveness of 8‐ or 12‐weeks of ledipasvir and sofosbuvir in real‐world treatment‐naive, genotype 1 hepatitis C infected patients. Aliment Pharmacol Ther. 2017;46:540–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

